677213
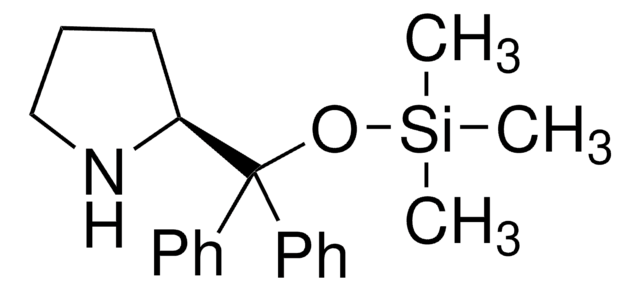
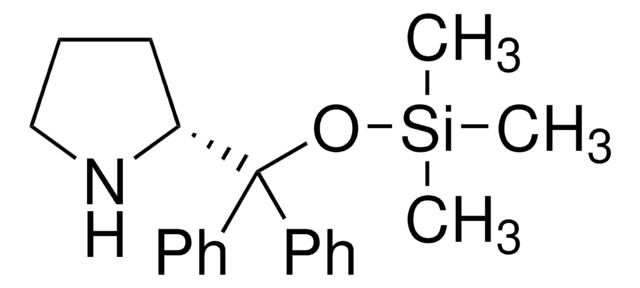
(R)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol trimethylsilyl ether
technical grade
Synonym(s):
(R)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]prolinol trimethylsilyl ether, (R)-2-[(Bis(3,5-bis(trifluoromethyl)phenyl)]trimethylsilanyloxy)methyl]pyrrolidine, (R)-2-[(Bis(3,5-bis(trifluoromethyl)phenyl)]trimethylsilyloxy)methyl]pyrrolidine
About This Item
Recommended Products
grade
technical grade
optical purity
enantiomeric excess: ≥99.0% (HPLC)
mp
46-55 °C
functional group
fluoro
SMILES string
C[Si](C)(C)OC([C@H]1CCCN1)(c2cc(cc(c2)C(F)(F)F)C(F)(F)F)c3cc(cc(c3)C(F)(F)F)C(F)(F)F
InChI
1S/C24H23F12NOSi/c1-39(2,3)38-20(19-5-4-6-37-19,13-7-15(21(25,26)27)11-16(8-13)22(28,29)30)14-9-17(23(31,32)33)12-18(10-14)24(34,35)36/h7-12,19,37H,4-6H2,1-3H3/t19-/m1/s1
InChI key
MOHRGTBNEJKFMB-LJQANCHMSA-N
Related Categories
Application
- Cyclocondensation of enals with methylenepyrrolidines
- Organocatalytic additions of β-ketosulfoxides to conjugated aldehydes
- Organocatalytic aza-Michael reactions
- Stereoselective propargylic alkylation of propargylic esters with aldehydes
- Epoxidation or aziridination of α,β-unsaturated aldehydes and Feist-Benary reactions of 1,3-dicarbonyls
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
>230.0 °F - closed cup
Flash Point(C)
> 110 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Professor Geoffrey Coates and co-workers at Cornell University have reported the preparation and use of catalysts composed of an oxophilic Lewis acid and a cobalt tetracarbonyl anion for the ring expansive carbonylation of epoxides to b-lactones and b-lactones to succinic anhydrides.
Professor Karl Anker Jørgensen and his group have developed ethers which serve as excellent chiral organocatalysts in the direct asymmetric α-functionalization of aldehydes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![(S)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol trimethylsilyl ether 97%](/deepweb/assets/sigmaaldrich/product/structures/396/398/09a397b1-b5f5-420f-98da-adf9017cef56/640/09a397b1-b5f5-420f-98da-adf9017cef56.png)


![(S)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol ≥99.0%](/deepweb/assets/sigmaaldrich/product/structures/201/440/11d18670-8609-4657-bb4b-af6c424f8791/640/11d18670-8609-4657-bb4b-af6c424f8791.png)

![(S)-2-[[3,5-Bis(trifluoromethyl)phenyl]thioureido]-N-benzyl-N,3,3-trimethylbutanamide 97%](/deepweb/assets/sigmaaldrich/product/structures/373/888/118b46f2-6c2e-4a87-8266-c4dbcd5db51f/640/118b46f2-6c2e-4a87-8266-c4dbcd5db51f.png)


