135321
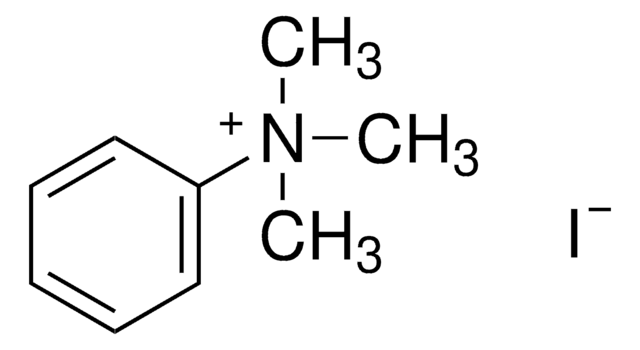
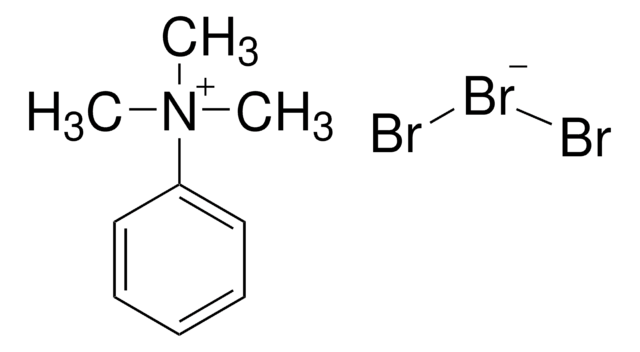
Trimethylphenylammonium bromide
98%
Synonym(s):
Phenyltrimethylammonium bromide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)3N(Br)C6H5
CAS Number:
Molecular Weight:
216.12
Beilstein:
3917006
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
technique(s)
titration: suitable
mp
215 °C (dec.) (lit.)
SMILES string
[Br-].C[N+](C)(C)c1ccccc1
InChI
1S/C9H14N.BrH/c1-10(2,3)9-7-5-4-6-8-9;/h4-8H,1-3H3;1H/q+1;/p-1
InChI key
GNMJFQWRASXXMS-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Neera Singh
Journal of environmental science and health. Part. B, Pesticides, food contaminants, and agricultural wastes, 41(1), 17-29 (2006-01-06)
The study aims to prepare the organoclay complexes of metolachlor and metribuzin so as to reduce their downward mobility in soil profile. The organoclays were preadsorbed with phenyltrimethylammonium (PTMA) (50% of cation exchange capacity [CEC]) and hexadecyltrimethylammonium (HDTMA) (100% of
Godfrey Lisk et al.
Molecular pharmacology, 71(5), 1241-1250 (2007-02-09)
Human red blood cells infected with the malaria parasite Plasmodium falciparum have markedly increased permeabilities to diverse organic and inorganic solutes. The plasmodial surface anion channel (PSAC), recently identified with electrophysiological methods, contributes to the uptake of many small solutes.
Alec N Salt et al.
Hearing research, 283(1-2), 14-23 (2011-12-20)
It has been widely believed that drug entry from the middle ear into perilymph occurs primarily via the round window (RW) membrane. Entry into scala vestibuli (SV) was thought to be dominated by local, inter-scala communication between scala tympani (ST)
D Monnaie et al.
Biochemistry, 39(18), 5349-5354 (2000-05-23)
In the process of characterizing the Na(+)-binding properties of factor Xa, a specific inhibition of this enzyme by quaternary amines was identified, consistent with previous observations. The binding occurs with K(i) in the low millimolar range, with trimethylphenylammonium (TMPA) showing
Jie Zhang et al.
Journal of chromatography. A, 1216(44), 7527-7532 (2009-04-07)
A method has been established for the determination of four pharmaceutically active compounds (ibuprofen, ketoprofen, naproxen and clofibric acid) in water samples using dynamic hollow fiber liquid-phase microextraction (HF/LPME) followed by gas chromatography (GC) injection port derivatization and GC-mass spectrometric
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service