A0664
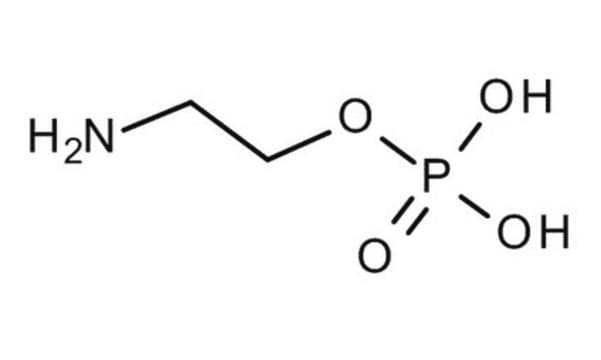
4-Aminobutylphosphonic acid
≥99%
Synonyme(s) :
P-(4-aminobutyl)-phosphonic acid
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C4H12NO3P
Numéro CAS:
Poids moléculaire :
153.12
Numéro MDL:
Code UNSPSC :
12352106
ID de substance PubChem :
Nomenclature NACRES :
NA.26
Produits recommandés
Niveau de qualité
Essai
≥99%
Forme
powder
Couleur
white
Application(s)
detection
Chaîne SMILES
NCCCCP(O)(O)=O
InChI
1S/C4H12NO3P/c5-3-1-2-4-9(6,7)8/h1-5H2,(H2,6,7,8)
Clé InChI
IDPXFPYGZRMMKZ-UHFFFAOYSA-N
Actions biochimiques/physiologiques
4-Aminobutylphosphonic acid, a GABA B receptor ligand, is used in studies on the regulation of prolactin (PRL) secretion and differential GABA receptor research.
4-aminobutylphosphonic acid, the phosphonic acid analogue of delta-aminovaleric acid, inhibits gamma-aminobutyric acid B (GABA-B receptor) binding without influencing either isoproterenol- or forskolin-stimulated cyclic AMP production.
Liaison
Analog of δ-aminovaleric acid
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
N G Ternan et al.
FEMS microbiology letters, 184(2), 237-240 (2000-03-14)
A strain of the yeast Kluyveromyces fragilis was screened for its ability to utilize a range of synthetic and natural organophosphonate compounds as the sole source of phosphorus, nitrogen or carbon. Only 4-aminobutylphosphonate was utilized as sole nitrogen source with
H P De Koning et al.
Endocrinology, 132(2), 674-681 (1993-02-01)
The activity of many endocrine cells is regulated by gamma-aminobutyric acid (GABA). The effects of GABA are mediated by GABAA and/or GABAB receptors. While GABAB receptors in the central nervous system have now been extensively characterized, little is known of
Oktay Yildirim et al.
International journal of molecular sciences, 11(3), 1162-1179 (2010-05-19)
FePt nanoparticles (NPs) were assembled on aluminum oxide substrates, and their ferromagnetic properties were studied before and after thermal annealing. For the first time, phosph(on)ates were used as an adsorbate to form self-assembled monolayers (SAMs) on alumina to direct the
L S Wong et al.
Pharmacology, biochemistry, and behavior, 38(4), 829-835 (1991-04-01)
The application of 1.2 and 12.0 micrograms/side of the GABAA receptor agonist 3-aminopropane sulphonic acid bilaterally into the nucleus accumbens (Acb) of rats nonsignificantly depressed locomotor activity as assessed in automated Animex activity cages, while the highest dose (60 micrograms/side)
N Tian et al.
Brain research, 660(2), 267-274 (1994-10-17)
Amacrine and ganglion cells in the amphibian retina contain GABAB, as well as GABAA, receptors. Baclofen, a GABAB agonist, hyperpolarizes the dark membrane potential of these third order neurons and makes their light responses more transient. GABAB receptors in the
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique