ABT1387
Anti-Phospho-Lamin A/C (Ser404)
from rabbit
Synonyme(s) :
Prelamin A/C, Renal carcinoma antigen NY-REN-32
About This Item
Produits recommandés
Source biologique
rabbit
Forme d'anticorps
affinity isolated antibody
Type de produit anticorps
primary antibodies
Clone
polyclonal
Espèces réactives
human
Technique(s)
immunofluorescence: suitable
inhibition assay: suitable (peptide)
western blot: suitable
Isotype
IgG
Numéro d'accès NCBI
Numéro d'accès UniProt
Modification post-traductionnelle de la cible
phosphorylation (pSer404)
Informations sur le gène
human ... LMNA(4000)
Description générale
Is cleaved to generate Lamin A/C. Farnesylation of prelamin-A/C facilitates nuclear envelope targeting and subsequent cleavage by ZMPSTE24/FACE1 to remove the farnesyl group produces mature Lamin-A/C that is inserted into the nuclear lamina. Lamin A and C are present in equal amounts in the lamina of mammals and they play an important role in nuclear assembly, chromatin organization, nuclear membrane and telomere dynamics. Lamins are shown to be essential for normal development of peripheral nervous system and skeletal muscle and for muscle satellite cell proliferation. Lamins also prevent fat infiltration of muscle and bone marrow, helping to maintain the volume and strength of skeletal muscle and bone. Phosphorylation of Lamins is reported to occur continuously throughout all interphase periods and takes place mainly on the assembled lamina. Phosphorylation of the major polypeptides of the lamina induces laminar disassembly during mitosis. Phosphorylated Lamin-A/C localizes to nucleoplasm. Lamin A/C undergoes phosphorylation at multiple sites and one of the best characterized phosphorylation sites is on Serine 22 and it is phosphorylated during interphase. Phosphorylation of Serine 22 stabilizes Lamin A/C. Overexpression of Lamin-A is shown to result in greater phosphorylation of Serine 22 and 390 and Lamin A/C knockdowns display reduced phosphorylation at both sites, which helps in maintaining the integrity of the diminished lamina. Lamin A/C can undergoes phosphorylation on Serine 404 by Akt1 and Ser4040 phosphorylated Lamin undergoes rapid lysosomal degradation. Mutations in LMNA gene can cause Emery-Dreifuss muscular dystrophy 2 and 3, which are characterized by weakness and atrophy of muscle without involvement of the nervous system and cardiac conduction defects. Some mutations have also been linked to familial Lipodystrophy that leads to the loss of subcutaneous adipose tissue in the lower parts of the body and accumulation of adipose tissue in the face and neck. (Ref.: Buxboim, A., et al. (2014). Curr. Biol. 24(16): 1909-1917; Toker, A., and Marmiroli, S. (2014). Adv. Biol. Regul. 55: 28-38).
Spécificité
Immunogène
Application
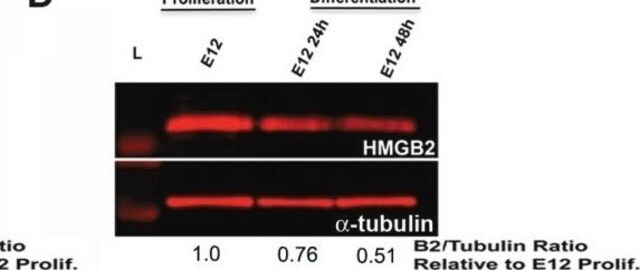
Peptide Inhibition Analysis: A 1:500 dilution from a representative lot was used with A549 cells (specific for Lamin A/C phosphorylation) for peptide block analysis.
Cell Structure
Qualité
Western Blotting Analysis: A 1:500 dilution of this antibody detected Phospho-Lamin A/C (Ser404) in A549 cells (specific for Lamin A/C phosphorylation).
Description de la cible
Forme physique
Stockage et stabilité
Autres remarques
Clause de non-responsabilité
Vous ne trouvez pas le bon produit ?
Essayez notre Outil de sélection de produits.
Code de la classe de stockage
12 - Non Combustible Liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique