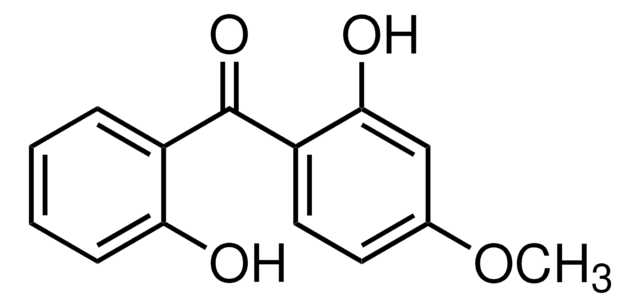
T16403
2,2′,4,4′-Tetrahydroxybenzophenone
97%
About This Item
Produits recommandés
Pureté
97%
Pf
198-200 °C (lit.)
Chaîne SMILES
Oc1ccc(c(O)c1)C(=O)c2ccc(O)cc2O
InChI
1S/C13H10O5/c14-7-1-3-9(11(16)5-7)13(18)10-4-2-8(15)6-12(10)17/h1-6,14-17H
Clé InChI
WXNRYSGJLQFHBR-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
- Urinary concentrations of bisphenol A, parabens and benzophenone-type ultra violet light filters in relation to sperm DNA fragmentation in young men: A chemical mixtures approach.: This study investigates the relationship between urinary concentrations of various chemicals, including benzophenone-type UV filters like 2,2′,4,4′-Tetrahydroxybenzophenone, and sperm DNA fragmentation. The findings highlight potential reproductive toxicity risks associated with exposure to these compounds (Kiwitt-Cárdenas et al., 2024).
- Salting-out assisted liquid-liquid extraction combined with LC-MS/MS for the simultaneous determination of seven organic UV filters in environmental water samples: method development and application.: This paper discusses the development of a method for detecting organic UV filters, including 2,2′,4,4′-Tetrahydroxybenzophenone, in environmental water samples. The method offers a reliable way to monitor the presence of these compounds in the environment (Carve et al., 2023).
- Novel visible-light-active P-g-CN-based α-Bi(2)O(3)/WO(3) ternary photocatalysts with a dual Z-scheme heterostructure for the efficient decomposition of refractory ultraviolet filters and environmental hormones: Benzophenones.: The study presents a new photocatalyst capable of degrading benzophenone-type UV filters, including 2,2′,4,4′-Tetrahydroxybenzophenone, under visible light. This development has significant implications for environmental cleanup technologies (Berekute et al., 2023).
- Boosting elimination of sunscreen, Tetrahydroxybenzophenone (BP-2), from water using monopersulfate activated by thorny NanoBox of Co@C prepared via the engineered etching strategy: A comparative and mechanistic investigation.: This research explores a novel method for removing 2,2′,4,4′-Tetrahydroxybenzophenone from water using advanced nanotechnology, highlighting its effectiveness and potential for environmental applications (Khiem et al., 2023).
- The binding mechanism of benzophenone-type UV filters and human serum albumin: The role of site, number, and type of functional group substitutions.: The study examines how benzophenone-type UV filters, including 2,2′,4,4′-Tetrahydroxybenzophenone, interact with human serum albumin, providing insights into their behavior in biological systems and potential health implications (Ma et al., 2023).
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Sens. 1A
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique