939358
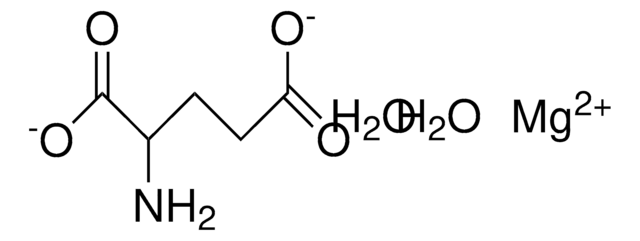
Magnesium acetate tetrahydrate

≥99.9% trace metals basis
Synonyme(s) :
Magnesium Diacetate Tetrahydrate,, Magnesium diethanoate tetrahydrate, Acetic acid magnesium salt
About This Item
Produits recommandés
Type
(High purity salts)
Niveau de qualité
Pureté
≥99.9% trace metals basis
98-102% (EDTA, complexometric)
Forme
powder or crystals
solid
Impuretés
<1000 ppm trace metal basis
Couleur
white to off-white
Pf
72-75 °C (lit.)
72-75 °C
Solubilité
water: soluble
Densité
1.454 g/cm3
Traces d'anions
chloride (Cl-): ≤20 ppm
sulfate (SO42-): <50 ppm
Traces de cations
Al: <50 ppm
K: <50 ppm
Mg: <100 ppm
Na: <50 ppm
Pb: <50 ppm
Zn: <50 ppm
Application(s)
battery manufacturing
Chaîne SMILES
O.O.O.O.CC(=O)O[Mg]OC(C)=O
InChI
1S/2C2H4O2.Mg.4H2O/c2*1-2(3)4;;;;;/h2*1H3,(H,3,4);;4*1H2/q;;+2;;;;/p-2
Clé InChI
XKPKPGCRSHFTKM-UHFFFAOYSA-L
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- A key components for the synthesis of spinel magnesium manganese oxide (Mg0.5Mn2.5O4 ) through one-step colloidal synthesis method. The nanocrystals has exhibited significant electrochemical activities in presence of diverse electrolytes.
- A starting materials for the production of Mesoporous (ZnO)x(MgO)1−x nanoplates by a template-free solvothermal synthetic method followed by subsequent calcination. These materials exhibit a band gap resulting from the presence of ZnO and MgO. The broad peaks observed in the 400-700 nm range indicate the presence of oxygen vacancy defects on the surface of the (ZnO)x(MgO)1−x nanoplates. These nanocrystals showed superior photocatalytic activities for the degradation of methyl orange (MO) in aqueous solution.
- To the synthesis of hydrophobic antireflective films of MgF2 with silicon modified with enhenced durability through sol-gel method.
- As a material for synthesizing carbon nanoribbons using ferrocene at high temperatures. The resulting nanoribbons exhibit a remarkably high surface area and demonstrate a stable reversible capacity of 750 mA h g−1 after 300 cycles in a charge-discharge experiment conducted at 0.5 A g−1. Due to these properties, it would be highly beneficial as an electrode material in electronic devices.
Caractéristiques et avantages
Medium purity (99.9%)
Low trace metals in ppm level
Cost effective
Low Chloride and sulfate levels
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique