647292
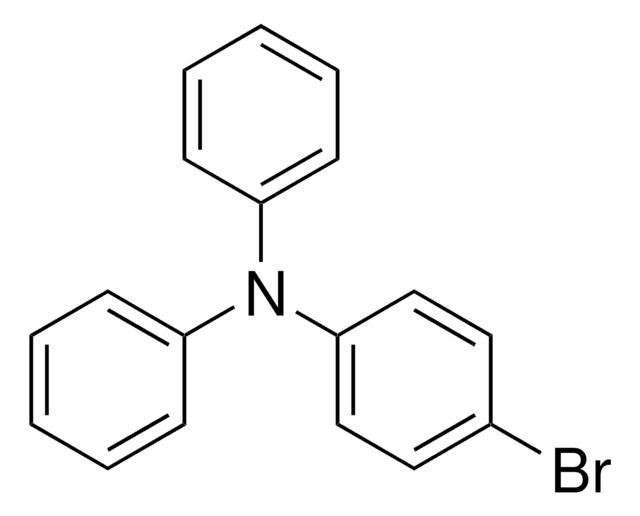
4-(Diphenylamino)phenylboronic acid
≥95%
Synonyme(s) :
4-(N,N-Diphenylamino)-1-phenylboronic acid, 4-(N,N-Diphenylamino)phenylboronic acid, 4-(N-Diphenylamino)phenylboronic acid, 4-(Diphenylamino)benzeneboronic acid, Triphenylamine-4-boronic acid
About This Item
Produits recommandés
Pureté
≥95%
Forme
powder
Pf
110-115 °C (lit.)
Chaîne SMILES
OB(O)c1ccc(cc1)N(c2ccccc2)c3ccccc3
InChI
1S/C18H16BNO2/c21-19(22)15-11-13-18(14-12-15)20(16-7-3-1-4-8-16)17-9-5-2-6-10-17/h1-14,21-22H
Clé InChI
TWWQCBRELPOMER-UHFFFAOYSA-N
Catégories apparentées
Application
- Strong multiphoton-excited blue photoluminescence and lasing from ladder-type oligo(p-phenylene)s
- Suzuki coupling reactions
- Ligand-free Suzuki reaction
Reagent used in Preparation of
- Push-pull arylvinyldiazine chromophores
- Benzothiadiazole-based fluorophores contg. triphenylamine functionality
- Blue light-emitting and hole-transporting materials for electroluminescent devices
- p-quaterphenyls laterally substituted with dimesitylboryl group for use as solid-state blue emitters
- Efficient sensitizers for dye-sensitized solar cells
- Prange electroluminescent materials for single-layer white polymer OLEDs
- Eeep-blue organic light emitting devices (OLEDs)
- Ligands for Organic Photovoltaic cells
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique![B-[4-(1,2,2-Triphenylethenyl)phenyl]boronic acid](/deepweb/assets/sigmaaldrich/product/structures/121/044/864e0829-e1de-4170-aae4-16c2b3ce4111/640/864e0829-e1de-4170-aae4-16c2b3ce4111.png)





![Benzo[b]thien-2-ylboronic acid ≥95%](/deepweb/assets/sigmaaldrich/product/structures/251/077/d0ead874-b533-4dcb-890d-8816a0018ccd/640/d0ead874-b533-4dcb-890d-8816a0018ccd.png)