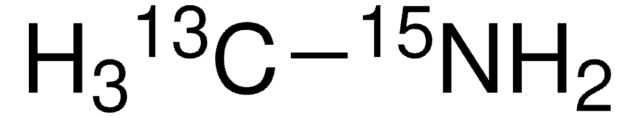490288
Methylamine-15N hydrochloride
98 atom % 15N
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule linéaire :
CH315NH2·HCl
Numéro CAS:
Poids moléculaire :
68.51
Numéro MDL:
Code UNSPSC :
12352116
ID de substance PubChem :
Nomenclature NACRES :
NA.12
Produits recommandés
Pureté isotopique
98 atom % 15N
Forme
solid
Pf
232-234 °C (lit.)
Changement de masse
M+1
Chaîne SMILES
Cl.C[15NH2]
InChI
1S/CH5N.ClH/c1-2;/h2H2,1H3;1H/i2+1;
Clé InChI
NQMRYBIKMRVZLB-CGOMOMTCSA-N
Catégories apparentées
Conditionnement
This product may be available from bulk stock and can be packaged on demand. For information on pricing, availability and packaging, please contact Stable Isotopes Customer Service.
Mention d'avertissement
Warning
Mentions de danger
Classification des risques
Acute Tox. 4 Oral
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
April D Lewoczko et al.
Physical chemistry chemical physics : PCCP, 15(13), 4707-4714 (2013-02-21)
Using density functional theory calculations, we report on the adsorption of methylamine on gold and compare its adsorption to a selection of alkylamines, methanol and methanethiol. On the (111) surface, the amines, thiol and alcohol bind in the ontop site
Thomas S Hofer et al.
Molecular bioSystems, 8(11), 2891-2900 (2012-08-01)
Molecular dynamics simulations have been performed to investigate the binding of tris(hydroxymethyl)-aminomethane to the surface of the core domain of the mouse cellular tumor antigen p53 employing the GROMOS and 53A6 force field parameter sets. A close investigation of the
Alejandro Cruz et al.
Molecules (Basel, Switzerland), 17(9), 10178-10191 (2012-08-28)
Symmetric and non-symmetric 2-(N-H, N-methyl, N-ethylenyl and N-aryl)guanidinebenzothiazoles were synthesized from the reaction of ammonia, methylamine, pyrrolidine and aniline with dimethyl benzo[d]thiazol-2-yl-carbonodithioimidate as intermediate. The products were characterized by ¹H-, ¹³C-NMR spectroscopy and three of them by X-ray diffraction analysis.
Yongqian Zhang et al.
Analytica chimica acta, 752, 106-111 (2012-10-30)
Both endogenous and exogenous methylamine have been found to be involved in many human disorders. The quantitative assessment of methylamine has drawn considerable interest in recent years. Although there have been many papers about the determination of methylamine, only a
Nicolas Fleury-Brégeot et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(31), 9564-9570 (2012-07-07)
Ammoniomethyl trifluoroborates are very powerful reagents that can be used to access biologically relevant aryl- and heteroaryl-methylamine motifs via Suzuki-Miyaura cross-couplings. Until now, this method was limited to the production of tertiary and primary amines. The synthesis of a large
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique