100625
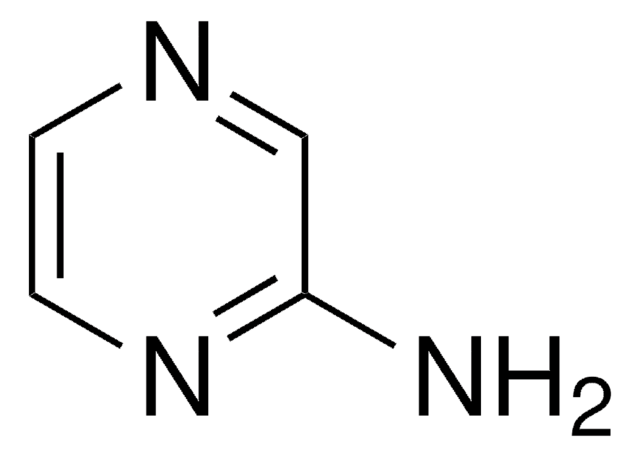
3-Amino-1,2,4-triazine
97%
Synonyme(s) :
3-Amino-as-triazine, [1,2,4]Triazin-3-ylamine
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C3H4N4
Numéro CAS:
Poids moléculaire :
96.09
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Pureté
97%
Pf
174-177 °C (lit.)
Chaîne SMILES
Nc1nccnn1
InChI
1S/C3H4N4/c4-3-5-1-2-6-7-3/h1-2H,(H2,4,5,7)
Clé InChI
MJIWQHRXSLOUJN-UHFFFAOYSA-N
Catégories apparentées
Description générale
3-amino-1,2,4-triazine (ATN) acts as a single molecular carbon nitride precursor during the preparation of mesoporous carbon nitride with high nitrogen content via polymerization. Additionally, it is also used in the synthesis of organometal complexes.
Application
- Antitumor activity in pharmaceutical applications: A study reported the development of a new library of 3-Amino-1,2,4-Triazine derivatives as PDK1 inhibitors, showing significant antitumor activity against pancreatic ductal adenocarcinoma, indicating its potential in cancer therapeutics (Carbone et al., 2023).
- Structural analysis in crystallography: The crystal structure and Hirshfeld surface analysis of a complex involving 3-Amino-1,2,4-triazine was detailed, providing insights into the molecular interactions and potential applications in materials science and coordination chemistry (Sangeetha et al., 2018).
- Catalytic applications in NMR technology: 3-Amino-1,2,4-triazine was used in a study to achieve significant NMR polarization in water using the SABRE technique, demonstrating its role as a catalyst in enhancing NMR sensitivity and its utility in magnetic resonance imaging (Zeng et al., 2014).
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Crystal structure of 3-amino-1,2,4-triazin-5(2H)-one.
Long-Chih Hwang et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 18(6), 723-724 (2002-06-27)
P A Taheri et al.
The Journal of clinical investigation, 93(1), 147-154 (1994-01-01)
Ultrasonic probes were placed around dog femoral arteries to record blood flow. Hind paw scalding with boiling water (5 s) caused a marked increase in ipsilateral femoral blood flow that persisted for the 2-h observation period. Contralateral femoral blood flow
S S Greenberg et al.
Life sciences, 57(21), 1949-1961 (1995-01-01)
We evaluated the effect of in vivo and in vitro administration of nitro-containing and nitro-deficient L-arginine-derived nitric oxide (NO) synthase inhibitors on the measurement of NO in plasma, urine and HEPES buffered physiologic salt solution (PSS) by ozone chemiluminescence and
Grant Abernethy et al.
Journal of chromatography. A, 1285, 165-167 (2013-03-12)
A rapid liquid chromatography-mass spectrometry method to detect 3-amino-1,2,4-triazine (ATZ) in milk was developed as part of a programme to set up methods for detecting the economically motivated adulteration of raw milk with nitrogen-containing compounds. When ATZ was added to
Jirí Hanusek et al.
Organic & biomolecular chemistry, 5(3), 478-484 (2007-01-26)
Contrary to a previous report, the sulfurisation of phosphorus(III) derivatives by 3-amino-1,2,4-dithiazole-5-thione (xanthane hydride) does not yield carbon disulfide and cyanamide as the additional reaction products. The reaction of xanthane hydride with triphenyl phosphine or trimethyl phosphite yields triphenyl phosphine
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique