07039
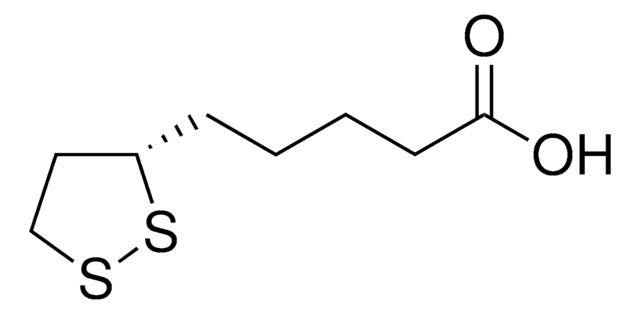
(R)-(+)-α-Lipoic acid
≥98.0% (HPLC)
Synonym(s):
(R)-(+)-1,2-Dithiolane-3-pentanoic acid, (R)-6,8-Dithiooctanoic acid, (R)-6,8-Thioctic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H14O2S2
CAS Number:
Molecular Weight:
206.33
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Quality Level
100
200
Assay
≥98.0% (HPLC)
optical purity
enantiomeric excess: ≥98.0% (HPLC)
mp
48-52 °C (lit.)
storage temp.
−20°C
SMILES string
OC(=O)CCCC[C@@H]1CCSS1
OC(=O)CCCC[C@@H]1CCSS1
InChI
1S/C8H14O2S2/c9-8(10)4-2-1-3-7-5-6-11-12-7/h7H,1-6H2,(H,9,10)/t7-/m1/s1
InChI key
AGBQKNBQESQNJD-SSDOTTSWSA-N
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
(R)-(+)-α-Lipoic acid was shown to significantly increase pyruvate oxidation while abrogating fatty acid oxidation in rat hepatocytes. These effects make R-(+)-α-Lipoic acid a promising treatment option for the treatment of Type II diabetes. It is a biological antioxidant with prooxidant activities, its therapeutic potential is widely investigated, e.g. in the treatment for Alzheimer′s disease.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Analysis Note
identity confirmed by LC-MS
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dove J Keith et al.
Pharmacological research, 66(3), 199-206 (2012-05-23)
Lipoic acid (LA) shows promise as a beneficial micronutrient toward improving elder health. Studies using old rats show that (R)-α-LA (R-LA) significantly increases low molecular weight antioxidants that otherwise decline with age. Despite this rationale for benefiting human health, little
Jennie L Walgren et al.
Metabolism: clinical and experimental, 53(2), 165-173 (2004-02-10)
R-(+)-alpha-lipoic acid (R-LA) is the naturally occurring enantiomer of LA. It is a strong antioxidant and cofactor of key metabolic enzyme complexes catalyzing the decarboxylation of alpha-keto acids. Racemic LA (rac-LA) has shown promise in treating diabetic polyneuropathy, and some
Ronald Bentley
Chemical Society reviews, 34(7), 609-624 (2005-06-21)
Chiral structures profoundly influence chemical and biological processes. While chiral carbon biomolecules have received much attention, chirality is also possible in certain sulfur compounds; just as with carbon, there can be differences in the physiological behavior of chiral sulfur compounds.
Takashi Yamada et al.
Neurochemistry international, 59(7), 1003-1009 (2011-10-13)
Growing evidence suggests that α-lipoic acid (LA) has neuroprotective effects in various pathological conditions including brain ischemia and neurodegeneration. While anti-oxidative activity has been thought to play a central role in LA-mediated neuroprotection, the precise mechanism and the effect of
L Packer et al.
Free radical biology & medicine, 19(2), 227-250 (1995-08-01)
alpha-Lipoic acid, which plays an essential role in mitochondrial dehydrogenase reactions, has recently gained considerable attention as an antioxidant. Lipoate, or its reduced form, dihydrolipoate, reacts with reactive oxygen species such as superoxide radicals, hydroxyl radicals, hypochlorous acid, peroxyl radicals
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service