19533
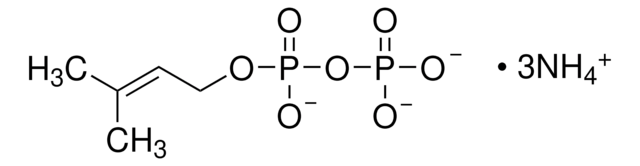
Geranyl pyrophosphate lithium salt
analytical standard
Synonym(s):
(E)-3,7-Dimethyl-2,6-octadien-1-ol trihydrogen pyrophosphate lithium salt, (E)-3,7-Dimethyl-2,6-octadien-1-yl pyrophosphate lithium salt, GPP-Li
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H20O7P2 · xLi+
CAS Number:
Molecular Weight:
314.21 (free acid basis)
Beilstein:
1915690
UNSPSC Code:
85151701
NACRES:
NA.24
Recommended Products
Quality Level
grade
analytical standard
Assay
≥95.0% (HPLC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
food and beverages
format
neat
shipped in
dry ice
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Application
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Analysis Note
NMR-Internal Standard : KHP
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Akira Saito et al.
Melanoma research, 18(2), 85-94 (2008-03-14)
Competitive inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase (the statins) that inhibit the synthesis of mevalonic acid are in wide use for treatment of hypercholesterolemia. Although antitumor effects on a variety of cell types have been reported for statins, the effect
Fu-Lien Hsieh et al.
Journal of molecular biology, 404(5), 859-873 (2010-10-23)
Isoprenoids, most of them synthesized by prenyltransferases (PTSs), are a class of important biologically active compounds with diverse functions. The mint geranyl pyrophosphate synthase (GPPS) is a heterotetramer composed of two LSU·SSU (large/small subunit) dimers. In addition to C(10)-GPP, the
Axel Schmidt et al.
Plant physiology, 152(2), 639-655 (2009-11-27)
The conifer Picea abies (Norway spruce) defends itself against herbivores and pathogens with a terpenoid-based oleoresin composed chiefly of monoterpenes (C(10)) and diterpenes (C(20)). An important group of enzymes in oleoresin biosynthesis are the short-chain isoprenyl diphosphate synthases that produce
Ken'ichi Hagiwara et al.
Biochemistry, 43(2), 300-309 (2004-01-14)
Farnesylation of the gamma-subunit of the retinal G-protein, transducin (Talpha/Tbetagamma), is indispensable for light-initiated signaling in photoreceptor cells. However, the farnesyl-mediated molecular interactions important for signaling are not well understood. To explore this issue, we created a functional Tbetagamma analogue
Tao-Hsin Chang et al.
The Plant cell, 22(2), 454-467 (2010-02-09)
Terpenes (isoprenoids), derived from isoprenyl pyrophosphates, are versatile natural compounds that act as metabolism mediators, plant volatiles, and ecological communicators. Divergent evolution of homomeric prenyltransferases (PTSs) has allowed PTSs to optimize their active-site pockets to achieve catalytic fidelity and diversity.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service










![Guanosine 5′-[β,γ-imido]triphosphate trisodium salt hydrate ≥85% (HPLC), powder](/deepweb/assets/sigmaaldrich/product/structures/204/494/05808804-1ca7-44bf-b6c5-d4934dc7cb85/640/05808804-1ca7-44bf-b6c5-d4934dc7cb85.png)