B75409
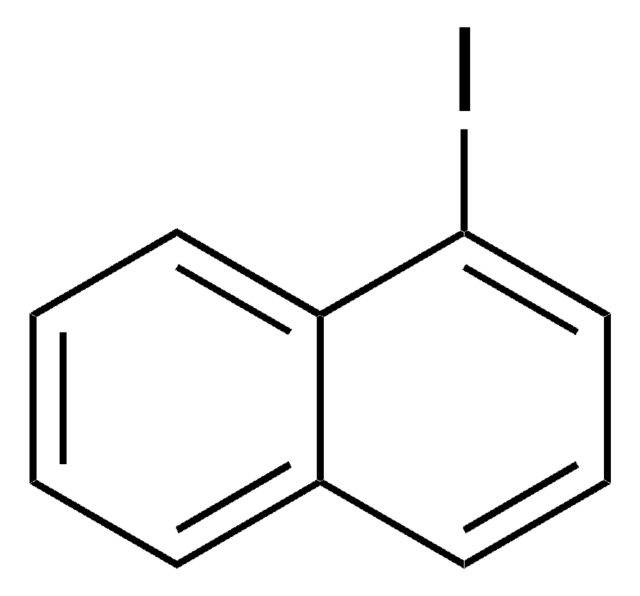
9-Bromophenanthrene
96%
Synonym(s):
9-Phenanthryl bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H9Br
CAS Number:
Molecular Weight:
257.13
Beilstein:
1869927
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
powder
bp
180-190 °C/2 mmHg (lit.)
mp
60-64 °C (lit.)
SMILES string
Brc1cc2ccccc2c3ccccc13
InChI
1S/C14H9Br/c15-14-9-10-5-1-2-6-11(10)12-7-3-4-8-13(12)14/h1-9H
InChI key
RSQXKVWKJVUZDG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
9-bromophenanthrene is a versatile halogenated organic compound that can be used as a reagent in various reactions such as Friedel-Crafts reaction and the Diels-Alder reaction. It is also used as a precursor for the synthesis of 9-bromoanthracene, 9-bromophenanthroline, and 9-bromophenanthridine.
Application
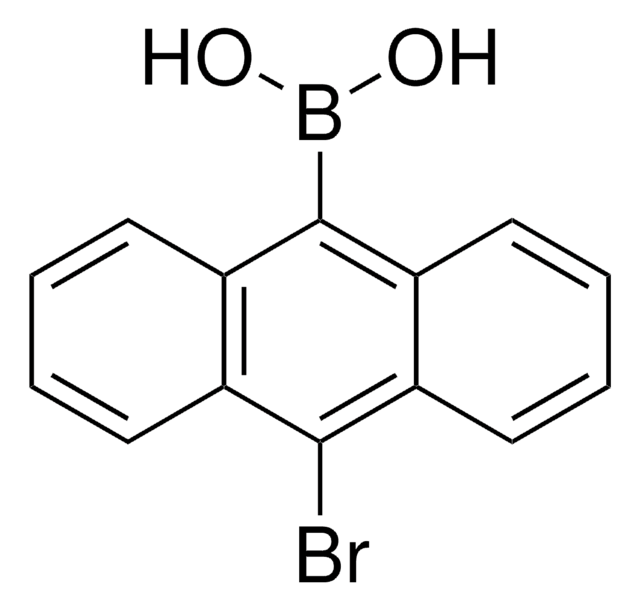
9-bromophenanthrene can be used as a building block in the synthesis of N-heterocyclic-carbene complexes via Suzuki−Miyaura cross-coupling reaction with aryl boronic acids.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Diels-Alder Addition of N, N-Diethyl-1, 3-butadienylamine to Dehydroaromatic Intermediates Generated from Some Haloaromatics (Commemoration Issue Dedicated to Professor Tatsuo Yamamoto on the Occasion of his Retirement)
Tanimoto
Bulletin of the Institute of Maritime and Tropical Medicine in Gdynia, 58, 289-292 (1980)
Aryne cycloaddition reactions in the synthesis of large polycyclic aromatic compounds
Perez, et al,
European Journal of Organic Chemistry, 2013, 5981-6013 (2013)
N-heterocyclic-carbene complexes readily prepared from di-$\mu$-hydroxopalladacycles catalyze the Suzuki arylation of 9-bromophenanthrene
Serrano, et al.
Organometallics, 34, 522-533 (2005)
Synthesis of 9, 9?-biphenanthryl-10, 10?-bis (oxazoline) s and their preliminary evaluations in the Friedel-Crafts alkylations of indoles with nitroalkenes
Lin S, et al.
Tetrahedron, 65, 1010-1016 (2009)
Charge-transfer complexes of brominated polycyclic aromatic hydrocarbons
Spotswood, T Mcl
Australian Journal of Chemistry, 15, 278-289 (1962)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service