862126
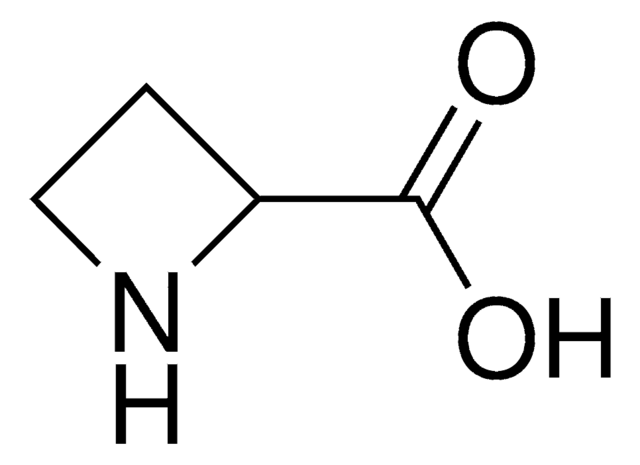
3,4-Dehydro-DL-proline
98%
Synonym(s):
(±)-2,5-Dihydro-1H-pyrrole-2-carboxylic acid, (±)-3-Pyrroline-2-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H7NO2
CAS Number:
Molecular Weight:
113.11
Beilstein:
471693
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
reaction suitability
reaction type: solution phase peptide synthesis
mp
245 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
OC(=O)C1NCC=C1
InChI
1S/C5H7NO2/c7-5(8)4-2-1-3-6-4/h1-2,4,6H,3H2,(H,7,8)
InChI key
OMGHIGVFLOPEHJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J B Cooper et al.
Plant physiology, 104(2), 747-752 (1994-02-01)
We investigated the function of cell wall hydroxyproline-rich glycoproteins by observing the effects of a selective inhibitor of prolyl hydroxylase, 3,4-dehydro-L-proline (Dhp), on wall regeneration by Nicotiana tabacum mesophyll cell protoplasts. Protoplasts treated with micromolar concentrations of Dhp do not
T A Sullivan et al.
The Journal of biological chemistry, 269(36), 22500-22506 (1994-09-09)
During development and fracture repair, endochondral bone formation is preceded by an orderly process of chondrocyte hypertrophy and cartilage matrix calcification. Analysis of calcifying versus noncalcifying cartilage has identified several differences in matrix proteins; among these are appearance of a
Ariel L L Garcia et al.
The Journal of organic chemistry, 70(3), 1050-1053 (2005-01-29)
We report herein a new, practical, and economic synthesis of the phosphodiesterase inhibitor Rolipram on a multigram scale as well as the synthesis of new 4-aryl pyrrolidones and beta-aryl-gamma-amino butyric acids (GABA derivatives) employing an efficient Heck-Matsuda arylation of 3-pyrroline
XueLian Zhang et al.
Journal of experimental botany, 59(14), 4045-4058 (2008-10-22)
Extensins are cell wall basic glycoproteins with a polypeptide backbone that is extremely rich in hydroxyproline. In this paper, the function of extensins in embryo development was studied in Nicotiana tabacum. By using Western blot and immunohistochemistry, the extensin JIM20
R W Leu et al.
Immunobiology, 188(3), 242-258 (1993-07-01)
Studies were designed to further define the modulatory role of complement subcomponent C1q in macrophage activation by Lipid A to mediate production of TNF and cytotoxic nitric oxide (NO). Pretreatment of macrophages for 24 h with 2.5 mM 3,4,dehydro-D,L-proline (DHP)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service