456764
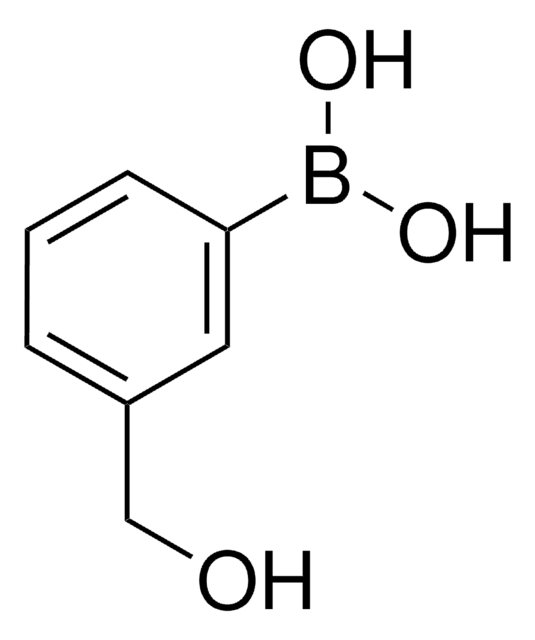
3-Carboxyphenylboronic acid
≥95%
Synonym(s):
μ-Carboxyphenylboronic acid, 3-(Dihydroxyborane)benzoic acid, 3-(Dihydroxyboryl)benzoic acid, 3-Boronobenzoic acid, 3-Carboxybenzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HO2CC6H4B(OH)2
CAS Number:
Molecular Weight:
165.94
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
mp
243-247 °C (lit.)
functional group
carboxylic acid
SMILES string
OB(O)c1cccc(c1)C(O)=O
InChI
1S/C7H7BO4/c9-7(10)5-2-1-3-6(4-5)8(11)12/h1-4,11-12H,(H,9,10)
InChI key
DBVFWZMQJQMJCB-UHFFFAOYSA-N
Application
3-Carboxyphenylboronic acid can be used as a substrate in the preparation of:
- Biaryl derivatives by reacting with bromoaniline through the Suzuki-Miyaura coupling reaction.
- Boronic acid-functionalized block copolymer.
- 1H-Imidazo[1,2-a]quinoxaline derivatives.
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Christopher G Barber et al.
Bioorganic & medicinal chemistry letters, 14(12), 3227-3230 (2004-05-20)
A series of 1-isoquinolinylguanidines are shown to be potent inhibitors of uPA with selectivity over tPA and plasmin. Potency is enhanced by the presence of a 4-halo and a 7-aryl substituent, particularly when substituted by a 3-carboxylic acid group. Compound
Heterocycles, 60, 1891-1897 (2003)
Novel rhodamine dyes via Suzuki coupling of xanthone triflates with arylboroxins
Calitree, B. D.; Detty, M. R.
Synlett, 89-92 (2010)
Di Wu et al.
Acta biomaterialia, 96, 123-136 (2019-06-28)
Locoregional chemotherapy, especially using implantable hydrogel depots to sustainably deliver chemotherapeutics at tumor site, has shown great potential for improving antitumor efficacy and reducing systemic toxicity. However, the hydrogel applications are limited by some intrinsic constraints, especially the contradiction between
Synthesis of a phenylboronic acid-functionalized thermosensitive block copolymer and its application in separation and purification of vicinal-diol-containing compounds
Wang Y, et al.
Royal Society of Chemistry Advances, 6(85), 82309-82320 (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service