440833
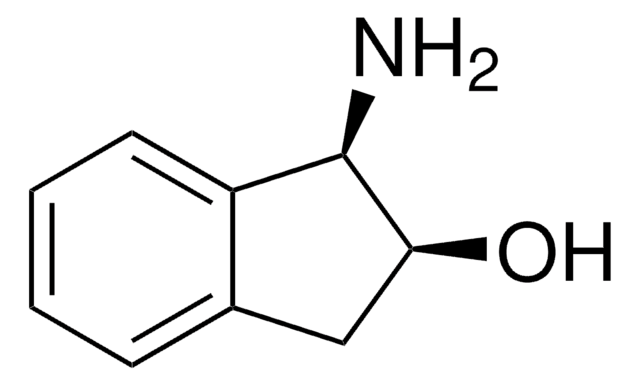
(1S,2R)-(−)-cis-1-Amino-2-indanol
99%
Synonym(s):
(1S,2R)-(−)-cis-1-Amino-2-hydroxyindane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H11NO
CAS Number:
Molecular Weight:
149.19
Beilstein:
4292559
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
optical purity
ee: 99% (GLC)
mp
118-121 °C (lit.)
functional group
hydroxyl
SMILES string
N[C@@H]1[C@H](O)Cc2ccccc12
InChI
1S/C9H11NO/c10-9-7-4-2-1-3-6(7)5-8(9)11/h1-4,8-9,11H,5,10H2/t8-,9+/m1/s1
InChI key
LOPKSXMQWBYUOI-BDAKNGLRSA-N
Looking for similar products? Visit Product Comparison Guide
General description
(1S,2R)-(-)-cis-1-Amino-2-indanol is a main constituent of indinavir, a potent HIV (human immunodeficiency virus) protease inhibitor.
Application
1S,2R)-(-)-cis-1-Amino-2-indanol may be used to prepare:
- (-)-1,2,5,6-Tetrahydropyridine by reacting with methyl (E,E)-4-oxo-2-[(2,6,6-trimethylcyclohex-1-enyl)vinyl}but-2-enoate.
- Oxazaborolidine catalysts, which can catalyze the asymmetric reduction of aromatic ketones with high enantioselectivity.
- (RS,1S,2R)-(-)-2,4,6-Trimethylbenzenesulfinic acid 1-(2,4,6-trimethylbenzenesulfonylamino)indan-2-yl ester.
Used to prepare a mannitol-based scaffold in the study of Plasmepsin II inhibition. Aspartic proteases such as plasmepsins I and II are of interest as targets for new, potential anti-malarials.
Physical properties
Useful chiral ligand for asymmetric synthesis.
Legal Information
Sold under license from Sterling Pharma Solutions Limited.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Karolina Ersmark et al.
Bioorganic & medicinal chemistry, 11(17), 3723-3733 (2003-08-07)
A series of C(2)-symmetric compounds with a mannitol-based scaffold has been investigated, both theoretically and experimentally, as Plm II inhibitors. Four different stereoisomers with either benzyloxy or allyloxy P1/P1' side chains were studied. Computational ranking of the binding affinities of
Improved asymmetric synthesis of aziridine 2-phosphonates using (S)-(+)-2, 4, 6-trimethylphenylsulfinamide.
Davis FA, et al.
The Journal of Organic Chemistry, 68(18), 6894-6898 (2003)
Cis-1-amino-2-indanol in asymmetric synthesis. Part I. A practical catalyst system for the enantioselective borane reduction of aromatic ketones.
Hong Y, et al.
Tetrahedron Letters, 35(36), 6631-6634 (1994)
Highly stereoselective asymmetric 6p-azaelectrocyclization utilizing the novel 7-alkyl substituted cis-1-amino-2-indanols: Formal synthesis of 20-epiuleine.
Tanaka K and Katsumura S.
Journal of the American Chemical Society, 124(33), 9660-9661 (2002)
Aldrichimica Acta, 31, 3-3 (1998)
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service