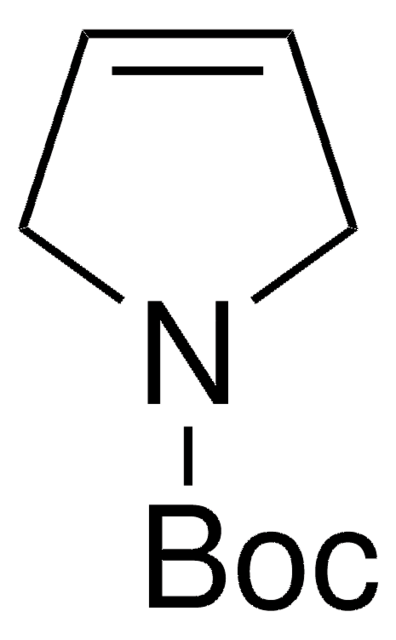
427055
N-Boc-pyrrolidine
97%
Synonym(s):
tert-Butyl 1-pyrrolidinecarboxylate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H17NO2
CAS Number:
Molecular Weight:
171.24
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.449 (lit.)
bp
80 °C/0.2 mmHg (lit.)
density
0.977 g/mL at 25 °C (lit.)
SMILES string
CC(C)(C)OC(=O)N1CCCC1
InChI
1S/C9H17NO2/c1-9(2,3)12-8(11)10-6-4-5-7-10/h4-7H2,1-3H3
InChI key
LPQZERIRKRYGGM-UHFFFAOYSA-N
General description
N-Boc-pyrrolidine is an N-substituted pyrrolidine. It is reported that the reactivity of N-Boc-pyrrolidine towards C-H insertion reaction is 2000 times more than cyclohexane. It undergoes α-arylation in the presence of a palladium catalyst with high enantioselectivity.
Application
N-Boc-pyrrolidine may be used in the synthesis of the following:
- 2-aryl-N-boc-pyrrolidines
- scalemic 2-pyrrolidinylcuprates
- 2-alkenyl-N-Boc-pyrrolidines
- 1-deoxycastanospermine
- methylphenidate analogues
- (+)-elaeokanine A
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
186.8 °F - closed cup
Flash Point(C)
86 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of methylphenidate analogues and their binding affinities at dopamine and serotonin transport sites.
Davies HML, et al.
Bioorganic & Medicinal Chemistry Letters, 14(7), 1799-1802 (2004)
Stereoselective synthesis of hydroxyindolizidines via sparteine-assisted deprotonation of N-Boc-pyrrolidine.
Majewski M, et al.
Tetrahedron Letters, 39(38), 6787-6790 (1998)
Copper mediated scalemic organolithium reagents in alkaloid syntheses.
3Dieter RK, et al.
Tetrahedron, 61(13), 3221-3230 (2005)
Enantioselective, palladium-catalyzed α-arylation of N-boc-pyrrolidine.
Campos KR, et al.
Journal of the American Chemical Society, 128(11), 3538-3539 (2006)
Catalytic asymmetric C-H activation by methyl thiophen-3-yldiazoacetate applied to the synthesis of (+)-cetiedil.
Davies HML, et al.
Tetrahedron Letters, 43(28), 4981-4983 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service