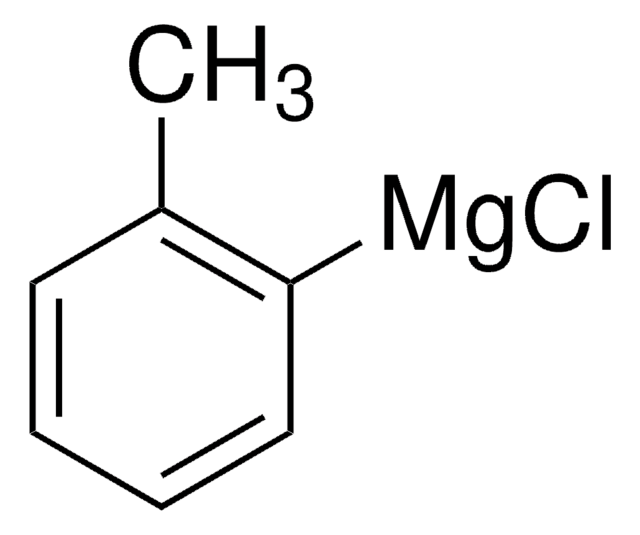
224448
Phenylmagnesium chloride solution
2.0 M in THF
Synonym(s):
Chlorophenylmagnesium
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5MgCl
CAS Number:
Molecular Weight:
136.86
Beilstein:
3587900
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
reaction suitability
reaction type: Grignard Reaction
concentration
2.0 M in THF
density
~1.02 g/mL at 20 °C
1.042 g/mL at 25 °C
SMILES string
Cl[Mg]c1ccccc1
InChI
1S/C6H5.ClH.Mg/c1-2-4-6-5-3-1;;/h1-5H;1H;/q;;+1/p-1
InChI key
GQONLASZRVFGHI-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
Phenylmagnesium chloride (PhMgCl) solution is a common Grignard reagent used in the synthesis of (−)-phenserine and stephacidin B. It is employed in a variety of cross-coupling reactions. It can also be used as an electrolyte solution along with aluminium chloride (AlCl3) in the rechargeable magnesium batteries.
Packaging
Other Notes
Storage below 25°C may cause formation of crystalline magnesium salts. Moving container to a warm location and occasional gentle swirling should redissolve the solid
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
1.4 °F - closed cup
Flash Point(C)
-17 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nickel-Catalyzed Cross-Coupling of Aryl Chlorides with Aryl Grignard Reagents.
B ohm VPW, et al.
Angewandte Chemie (International Edition in English), 39(9), 1602-1604 (2000)
Electrolyte solutions with a wide electrochemical window for rechargeable magnesium batteries.
Mizrahi O, et al.
Journal of the Electrochemical Society, 155(2), A103-A109 (2008)
Asymmetric synthesis of pyrrolidinoindolines. Application for the practical total synthesis of (−)-phenserine
Huang A, et al.
Journal of the American Chemical Society, 126(43), 14043-14053 (2004)
Enantioselective synthesis of stephacidin B.
Herzon S, et al.
Journal of the American Chemical Society, 127(15), 5342-5344 (2005)
Functional group tolerant Kumada- Corriu- Tamao coupling of nonactivated alkyl halides with aryl and heteroaryl nucleophiles: Catalysis by a nickel pincer complex permits the coupling of functionalized Grignard reagents.
Vechorkin O, et al.
Journal of the American Chemical Society, 131(28), 9756-9766 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service