116130
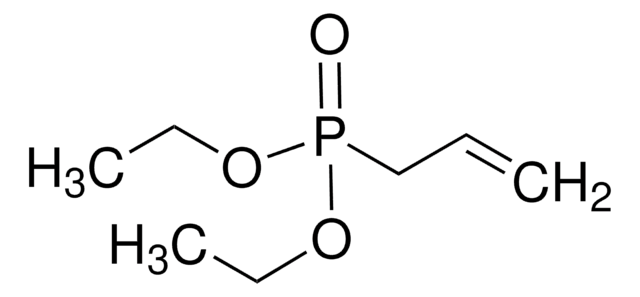
Diethyl vinylphosphonate
97%
Synonym(s):
Vinylphosphonic acid diethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH2=CHPO(OCH2CH3)2
CAS Number:
Molecular Weight:
164.14
Beilstein:
507596
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.429 (lit.)
bp
202 °C (lit.)
density
1.068 g/mL at 25 °C (lit.)
functional group
phosphonate
storage temp.
2-8°C
SMILES string
CCOP(=O)(OCC)C=C
InChI
1S/C6H13O3P/c1-4-8-10(7,6-3)9-5-2/h6H,3-5H2,1-2H3
InChI key
DREPONDJUKIQLX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
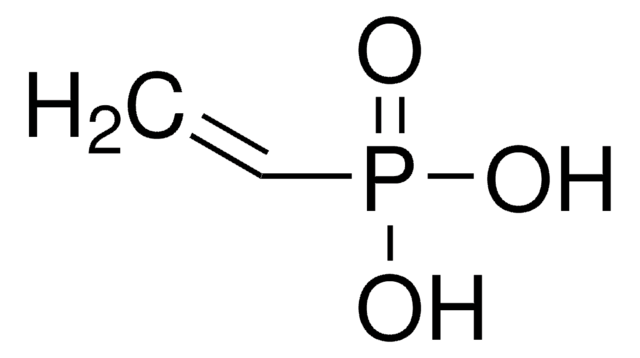
Diethyl vinylphosphonate (DEVP) can be used as a precursor for the synthesis of:
It can be also employed as a monomer unit for the preparation of high-molecular-weight polymer, poly(diethyl vinylphosphonate) using lanthanide complexes.{18)
- α, β-unsaturated phosphonates by reacting with arylboronic acids via Pd-catalyzed Mizoroki−Heck reaction.
- 2-(arylamino)ethyl phosphonates by condensing with primary and secondary amines via the aza-Michael addition reaction.
It can be also employed as a monomer unit for the preparation of high-molecular-weight polymer, poly(diethyl vinylphosphonate) using lanthanide complexes.{18)
Diethyl vinylphosphonate has been used in the preparation of diethyl N-alkyl-2-aminoethylphosphonate.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Convenient synthesis of α, β-unsaturated phosphonates via a Mizoroki-Heck reaction of arylboronic acids with diethyl vinylphosphonate
Kabalka GW, et al.
Tetrahedron Letters, 45(24), 4685-4687 (2004)
Synthesis of 2-(arylamino) ethyl phosphonic acids via the aza-Michael addition on diethyl vinylphosphonate
Orm Nadine B, et al.
Tetrahedron, 69(1), 115-121 (2013)
Joachim E Klee et al.
Beilstein journal of organic chemistry, 5, 72-72 (2009-01-01)
Novel N-alkyl-N-(phosphonoethyl) substituted mono-, bis- and tris(meth)acrylamides 3 were synthesized by two different three-step reactions and characterized by IR, (1)H NMR and (13)C NMR spectroscopy as well as refractive index and viscosity. The phosphonoethyl substituted (meth)acrylamide monomers show improved hydrolytic
Poly (vinylphosphonate) s synthesized by trivalent cyclopentadienyl lanthanide-induced group transfer polymerization
Salzinger S, et al.
Macromolecules, 44(15), 5920-5927 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![Bis[2-(methacryloyloxy)ethyl] phosphate](/deepweb/assets/sigmaaldrich/product/structures/128/336/4e7a3e38-338c-423e-95b8-70d9d1f8e121/640/4e7a3e38-338c-423e-95b8-70d9d1f8e121.png)


