All Photos(1)
About This Item
Empirical Formula (Hill Notation):
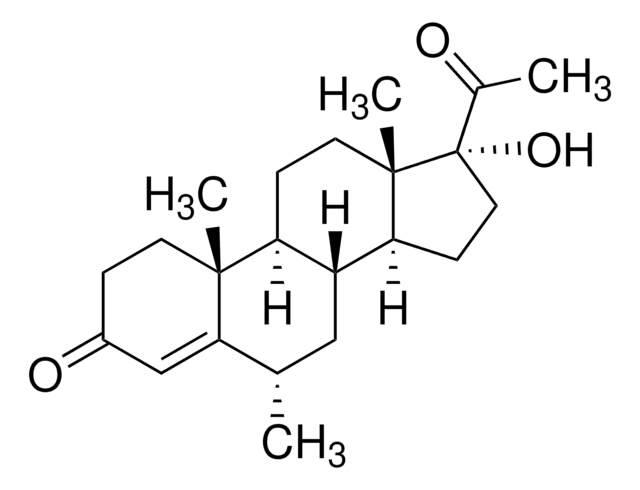
C20H28O2
CAS Number:
Molecular Weight:
300.44
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
form:
powder
Assay:
≥98% (TLC)
Recommended Products
Assay
≥98% (TLC)
form
powder
color
off-white
solubility
chloroform: 50 mg/mL, clear to slightly hazy, colorless to light yellow
shipped in
ambient
storage temp.
room temp
SMILES string
[H][C@]12CCC(=O)C=C1CC[C@]3([H])[C@]2([H])CC[C@]4(C)[C@H](CC[C@@]34[H])C(C)=O
InChI
1S/C20H28O2/c1-12(21)18-7-8-19-17-5-3-13-11-14(22)4-6-15(13)16(17)9-10-20(18,19)2/h11,15-19H,3-10H2,1-2H3/t15-,16+,17+,18+,19-,20+/m0/s1
InChI key
NVUUMOOKVFONOM-GPBSYSOESA-N
Gene Information
human ... SERPINA6(866)
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J Botella et al.
The Journal of steroid biochemistry and molecular biology, 55(1), 77-84 (1995-10-01)
Estrogen receptors of human endometrial cancer Ishikawa cells were found to be present in moderate amounts (160-200 fmol/mg protein), and to specifically bind moxestrol (R2858) with a very high affinity characterized by a Kd around 60 pM, when measured under
J Botella et al.
Journal de pharmacologie, 17(4), 699-706 (1986-10-01)
Structure-activity relationships were studied in vitro on a number of natural and artificial steroids in order to assess their progestagen specificity. These substances included a new compound derived from 19 nor progesterone, TX066 or nomegestrol acetate, and two synthetic intermediates
L Qi-Gui et al.
European journal of drug metabolism and pharmacokinetics, 16(2), 93-102 (1991-04-01)
Five synthetic progestins of the 19-nortestosterone type (norethisterone, NET; levonorgestrel, LN; gestodene, GEST; NET-3-oxime, NETO; norgestimate, NGM) were investigated in the in vitro hepatocyte model. Radiolabelled progestins were added to hepatocyte suspensions (3 x 10(6) cells/ml) freshly prepared from female
C E Hall et al.
Endocrinology, 109(4), 1168-1175 (1981-10-01)
The mineralocorticoid potency of 19-nor-progesterone was evaluated by both its effect on electrolyte excretion in adrenalectomized animals and its ability to cause hypertension and electrolyte changes in mononephrectomized, salt-loaded rats. The mineralocorticoid activity, measured using an adrenalectomized rat bioassay, indicated
M J Schiff et al.
Endocrinology, 115(4), 1235-1238 (1984-10-01)
19-Nor-corticosteroids are potentially important mineralocorticoids and hypertensive agents. We tested the mineralocorticoid potency of 19-nor-progesterone (19-NOR-P) and 19-nor-corticosterone (19-NOR-B) compared with aldosterone using the toad bladder short-circuit current as a measure of sodium transport. 19-NOR-B (10(-7) M) increased sodium transport
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service