W237701
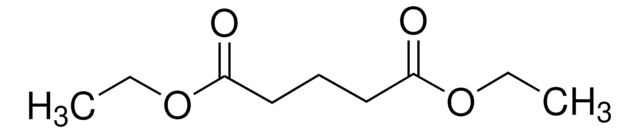
Diethyl succinate
≥99%, FCC, FG
Synonym(s):
Diethyl butanedioate
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
FG
Fragrance grade
Halal
Kosher
Agency
follows IFRA guidelines
meets purity specifications of JECFA
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 117
FDA 21 CFR 172.515
vapor density
6 (vs air)
Assay
≥99%
refractive index
n20/D 1.42 (lit.)
bp
218 °C (lit.)
mp
−20 °C (lit.)
density
1.047 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
Organoleptic
apple; grape; fruity; waxy; floral
SMILES string
CCOC(=O)CCC(=O)OCC
InChI
1S/C8H14O4/c1-3-11-7(9)5-6-8(10)12-4-2/h3-6H2,1-2H3
InChI key
DKMROQRQHGEIOW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Impact of indigenous Oenococcus oeni and Lactiplantibacillus plantarum species co-culture on Cabernet Sauvignon wine malolactic fermentation: Kinetic parameters, color, and aroma.: This study explores the co-culture fermentation process in Cabernet Sauvignon wine, focusing on kinetic parameters, color, and aroma. Diethyl succinate was analyzed among the volatile compounds, contributing to the wine′s aroma profile. (Zhang et al., 2024).
- Enhancement of ester biosynthesis in blueberry wines through co-fermentation via cell-cell contact between Torulaspora delbrueckii and Saccharomyces cerevisiae.: The research investigates the role of cell-cell contact in ester biosynthesis during the co-fermentation of blueberry wines. Diethyl succinate is one of the key esters produced, enhancing the aroma and flavor profile of the wine. (Wang et al., 2024).
- Enhancing blueberry wine aroma: Insights from cultivar selection and berry sorting.: This article examines how different blueberry cultivars and sorting methods impact the aroma of blueberry wine, with a focus on the production of aromatic esters such as diethyl succinate. (Wang et al., 2023).
- Comparative analysis of microbial communities and volatile flavor components in the brewing of Hongqu rice wines fermented with different starters.: The study provides a comparative analysis of microbial communities and their impact on volatile flavor components, including diethyl succinate, in Hongqu rice wines fermented with different starters. (Chen et al., 2023).
- Update to RIFM fragrance ingredient safety assessment, diethyl succinate, CAS Registry Number 123-25-1.: This update focuses on the safety assessment of diethyl succinate as a fragrance ingredient, providing comprehensive toxicological data and usage guidelines. (Api et al., 2023).
Biochem/physiol Actions
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
208.4 °F - closed cup
Flash Point(C)
98 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service