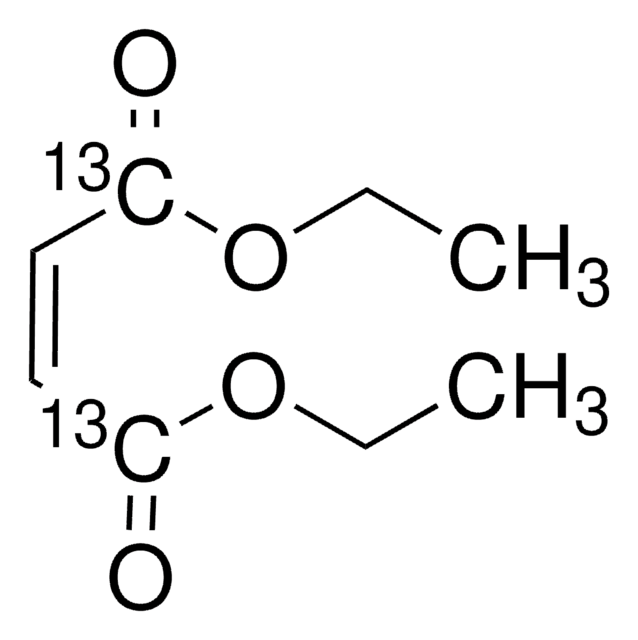
D95654
Diethyl fumarate
98%
Synonym(s):
(2E)-2-Butenedioic acid diethyl ester, (E)-But-2-enedioic acid diethyl ester, Diethyl (E)-but-2-enedioate, Diethyl fumaric acid ester, Ethyl fumarate
About This Item
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.44 (lit.)
bp
218-219 °C (lit.)
mp
1-2 °C (lit.)
density
1.052 g/mL at 25 °C (lit.)
SMILES string
[H]\C(=C(\[H])C(=O)OCC)C(=O)OCC
InChI
1S/C8H12O4/c1-3-11-7(9)5-6-8(10)12-4-2/h5-6H,3-4H2,1-2H3/b6-5+
InChI key
IEPRKVQEAMIZSS-AATRIKPKSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Relaxation and Amorphous Structure of Polymers: This study examines the behavior of polymers with rigid fumarate segments, including diethyl fumarate, and their impact on the relaxation and amorphous structures, enhancing polymer design and processing (Suzuki et al., 2022).
- Gradient versus End-Capped Degradable Polymer Sequence Variations: The research explores the mechanical properties of photochemically 3D-printed substrates using diethyl fumarate-based polymers, presenting new possibilities in creating tailored biomaterials for medical applications (Shin and Becker, 2022).
- Novel Curcumin-Diethyl Fumarate Hybrid for Parkinson′s Disease: This study investigates a hybrid molecule combining curcumin and diethyl fumarate, highlighting its dual function as a GSK-3β inhibitor and Nrf2 inducer, which could be significant for Parkinson′s disease treatment strategies (Di Martino et al., 2020).
- Synthesis and 3D Printing of PEG-Poly(propylene fumarate) Copolymers: This article describes the synthesis and application of diethyl fumarate in the creation of novel diblock and triblock copolymer hydrogels for 3D printing, expanding the utility in biomedicine and tissue engineering (Dilla et al., 2018).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
197.6 °F - closed cup
Flash Point(C)
92 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service