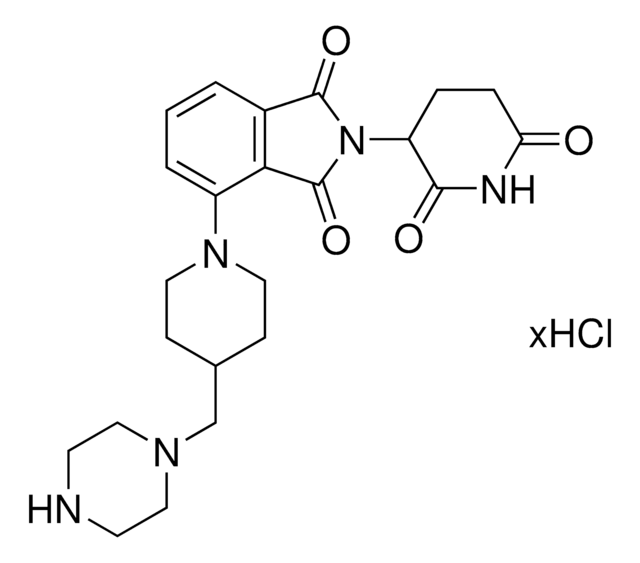
911771
Lenalidomide-Photoswitch1-NH2 hydrochloride
≥95%
Synonym(s):
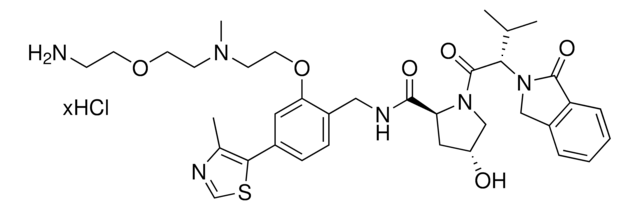
(E)-N-(4-Aminobutyl)-2-(4-((2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)diazenyl)-2,6-dimethoxyphenoxy)acetamide hydrochloride, PHOTAC template, Photoswitchable protein degrader building block for PROTAC® research
About This Item
Recommended Products
ligand
lenalidomide
Quality Level
Assay
≥95%
form
powder or crystals
reaction suitability
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
availability
available only in USA
functional group
amine
storage temp.
2-8°C
SMILES string
NCCCCNC(COC1=C(OC)C=C(/N=N/C2=CC=CC3=C2CN(C4C(NC(CC4)=O)=O)C3=O)C=C1OC)=O.Cl
Related Categories
Application
Suggested wavelengths for photoswitching:
- Switch to cis isomer: 390 nm (380-400 nm)
- Switch to trans isomer (thermally more stable isomer): >450 nm
Low-intensity light needed for photoactivation is not cytotoxic.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Learn more:
Other Notes
Legal Information
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service