All Photos(2)
About This Item
Empirical Formula (Hill Notation):
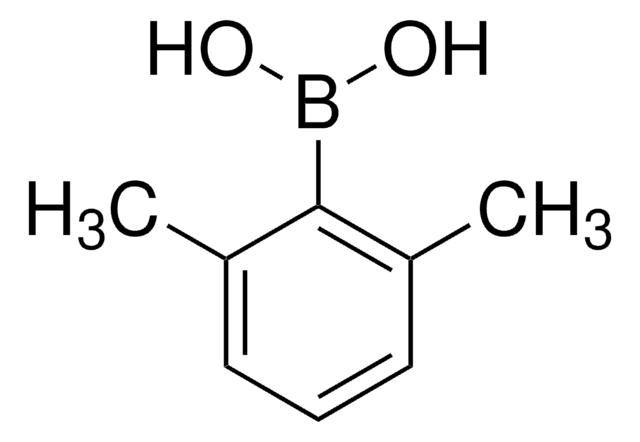
C12H19BO2
CAS Number:
Molecular Weight:
206.09
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
mp
167-172 °C
SMILES string
CC(C)c1cccc(C(C)C)c1B(O)O
InChI
1S/C12H19BO2/c1-8(2)10-6-5-7-11(9(3)4)12(10)13(14)15/h5-9,14-15H,1-4H3
InChI key
UPXGMXMTUMCLGD-UHFFFAOYSA-N
Application
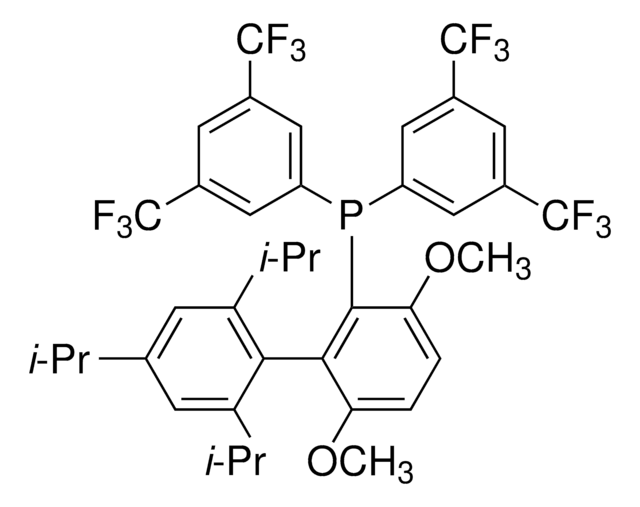
2,6-Diisopropylphenylboronic acid can be used as a reactant:
- In the Pd-catalyzed Suzuki-Miyaura cross-coupling reactions.
- To synthesis potent propargyl-based inhibitors of the Cryptosporidium dihydrofolate reductase (DHFR) enzyme.
- To prepare boralumoxane applicable in the activation of zirconocene catalyst for the polymerization ethene.
Storage Class Code
11 - Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and structural characterisation of a boralumoxane capable of activating a zirconocene ethene polymerisation catalyst
Richter B, et al.
Chemical Communications (Cambridge, England), (14), 1286-1287 (2001)
David B Bolstad et al.
Journal of medicinal chemistry, 51(21), 6839-6852 (2008-10-07)
Cryptosporidiosis is an emerging infectious disease that can be life-threatening in an immune-compromised individual and causes gastrointestinal distress lasting up to 2 weeks in an immune-competent individual. There are few therapeutics available for effectively treating this disease. We have been
Catalytic hydrosilylation of alkenes by iron complexes containing terpyridine derivatives as ancillary ligands
Kamata K, et al.
Organometallics, 31(10), 3825-3828 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)