65420
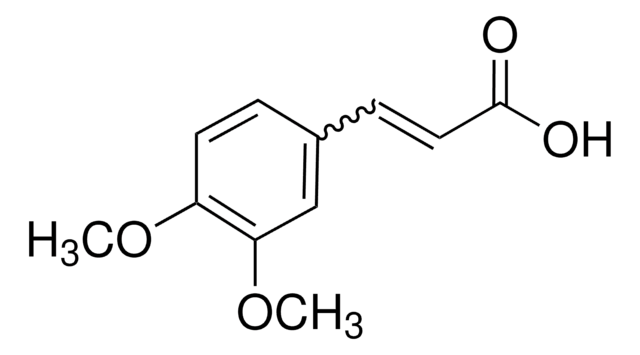
4-Methoxycinnamic acid
≥98.0% (GC)
Synonym(s):
trans-3-(4-Methoxyphenyl)acrylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H10O3
CAS Number:
Molecular Weight:
178.18
Beilstein:
2208397
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.0% (GC)
mp
173.5 °C (lit.)
SMILES string
COc1ccc(\C=C\C(O)=O)cc1
InChI
1S/C10H10O3/c1-13-9-5-2-8(3-6-9)4-7-10(11)12/h2-7H,1H3,(H,11,12)/b7-4+
InChI key
AFDXODALSZRGIH-QPJJXVBHSA-N
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yu-An Chen et al.
Bioresource technology, 269, 329-338 (2018-09-09)
The aim of this work was to study the effects of pretreatment process, hydrolysis condition and structural features of lignin on the improving action of surfactants (Tween 20) for enzymatic hydrolysis of pretreated wheat straw, and further to interpret the
Sirichai Adisakwattana et al.
International journal of molecular sciences, 13(2), 1778-1789 (2012-03-13)
Cinnamic acid and its derivatives have shown a variety of pharmacologic properties. However, little is known about the antiglycation properties of cinnamic acid and its derivatives. The present study sought to characterize the protein glycation inhibitory activity of cinnamic acid
Victor S Sobolev et al.
Journal of agricultural and food chemistry, 54(10), 3505-3511 (2006-05-17)
The peanut plant (Arachis hypogaea) is known to produce stilbene phytoalexins as a defensive response to fungal invasion; however, the distribution of phytoalexins among different organs of the peanut plant at early stages of growth under axenic conditions has not
H M Chawla et al.
Journal of photochemistry and photobiology. B, Biology, 105(1), 25-33 (2011-08-02)
A series of novel calix[4]arene enones (5-7) and cinnamates (12-14) have been synthesized and evaluated for ensuring protection from ultraviolet radiation (UVR). Spectroscopic analyses has revealed that compound 6 absorbs ultraviolet radiations between 280 and 350 nm with an absorption
So Ra Kim et al.
British journal of pharmacology, 135(5), 1281-1291 (2002-03-06)
1. We previously reported that four new phenylpropanoid glycosides and six known cinnamate derivatives isolated from roots of Scrophularia buergeriana Miquel (Scrophulariaceae) protected cultured cortical neurons from neurotoxicity induced by glutamate. Here, we have investigated the structure-activity relationships in the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service