All Photos(1)
About This Item
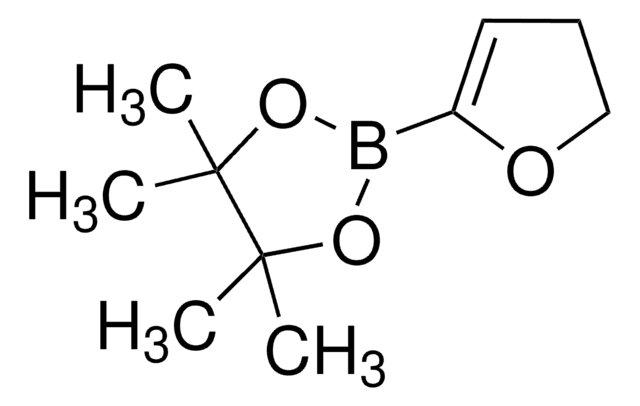
Linear Formula:
ClC6H3(F)I
CAS Number:
Molecular Weight:
256.44
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.604 (lit.)
bp
94-95 °C/15 mmHg (lit.)
density
2.008 g/mL at 25 °C (lit.)
SMILES string
Fc1ccc(I)cc1Cl
InChI
1S/C6H3ClFI/c7-5-3-4(9)1-2-6(5)8/h1-3H
InChI key
OMASDGWBVAVFQZ-UHFFFAOYSA-N
General description
3-Chloro-4-fluoroiodobenzene, also known as 2-chloro-1-fluoro-4-iodobenzene [IUPAC name], is a polyhalobenzene. It participates in the synthesis of 3-carboxythiophene analogs.
Application
3-Chloro-4-fluoroiodobenzene may be used in the preparation of 2-chloro-1-(3,5-dimethoxyphenoxy)-4-iodobenzene by reacting with 3,5-dimethoxyphenol in the presence of cesium carbonate.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Fused thiophene derivatives as MEK inhibitors
Laing VE, et al.
Bioorganic & Medicinal Chemistry Letters, 22.1 , 472-475 (2012)
Selectivity issues in the catalytic multiphase reduction of functionalized halogenated aromatics over Pd/C, Pt/C, and Raney-Ni
Evdokimova, Galina, et al.
Applied Catalysis A: General, 271.1, 129-136 (2004)
Synthesis of Phloroglucinol Monoaryl Ethers
Sherwood AM, et al.
Synthesis, 44.08, 1208-1212 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service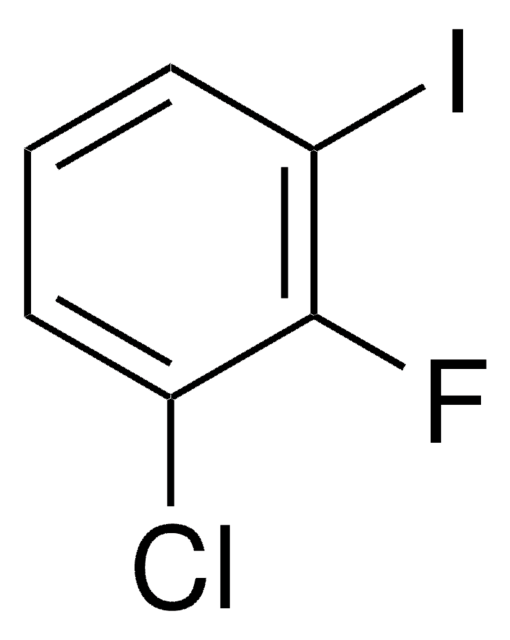


![6-Oxaspiro[2.5]octane-1-carboxylic acid AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/116/265/092e6253-e2e1-47f3-9513-19bf7d749365/640/092e6253-e2e1-47f3-9513-19bf7d749365.png)