All Photos(1)
About This Item
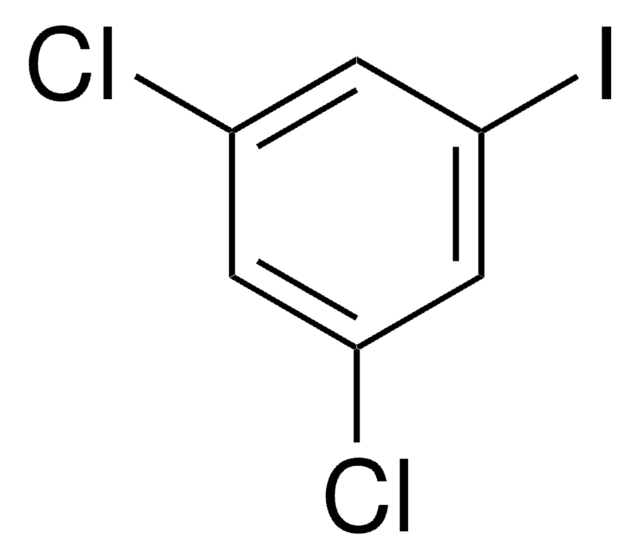
Linear Formula:
Cl2C6H3I
CAS Number:
Molecular Weight:
272.90
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
27-29 °C (lit.)
SMILES string
Clc1ccc(I)cc1Cl
InChI
1S/C6H3Cl2I/c7-5-2-1-4(9)3-6(5)8/h1-3H
InChI key
NADPFZNWCQIJJW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3,4-Dichloroiodobenzene undergoes Stille coupling reaction with vinyltributyltin in the presence of Pd(0)precursors to furnish styrene derivative.
Application
3,4-Dichloroiodobenzene may be used in the preparation of:
- tetrachloromethoxybiphenyls
- 1-(3,4-dichlorophenyl)-2-trimethylsilylacetylene
- 2-(3,4-dichlorocinnamyl)isoindoline-1,3-dione
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Cara A Mosley et al.
Bioorganic & medicinal chemistry, 17(17), 6463-6480 (2009-08-04)
The synthesis and structure-activity relationship analysis of a novel class of amide-based biaryl NR2B-selective NMDA receptor antagonists are presented. Some of the studied compounds are potent, selective, non-competitive, and voltage-independent antagonists of NR2B-containing NMDA receptors. Like the founding member of
Identification of chlorinated methoxybiphenyls as contaminants in fish and as potential interferences in the determination of chlorinated dibenzo-p-dioxins.
D W Phillipson et al.
Analytical chemistry, 52(14), 2328-2332 (1980-12-01)
Synthesis and properties of various poly (diphenylacetylenes) containing tert-amine moieties.
Katsumata T, et al.
Polymer, 49(12), 2808-2816 (2008)
Leonardo S Santos et al.
The Journal of organic chemistry, 72(15), 5809-5812 (2007-06-26)
On-line monitoring of Stille reactions was performed via direct infusion electrospray ionization mass spectrometry (ESI-MS) and its tandem version (ESI-MS/MS). When operated in the positive ion mode, ESI(+)-MS was able to transfer, directly from solution to the gas phase, the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service