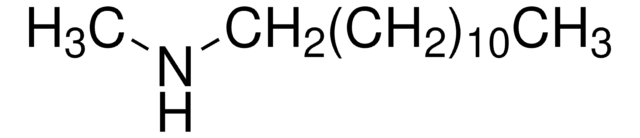All Photos(2)
About This Item
Linear Formula:
[CH3(CH2)5]2NCH3
CAS Number:
Molecular Weight:
199.38
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97%
refractive index
n20/D 1.433 (lit.)
bp
146 °C/48 mmHg (lit.)
density
0.775 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CCCCCCN(C)CCCCCC
InChI
1S/C13H29N/c1-4-6-8-10-12-14(3)13-11-9-7-5-2/h4-13H2,1-3H3
InChI key
POMGZMHIXYRARC-UHFFFAOYSA-N
General description
N-Methyldihexylamine (dihexylmethylamine) is a tertiary amine. It is formed during the pyrolysis of 1,1-dihexyl-1-methylamine-2-acylimide.
Application
N-Methyldihexylamine may be used to synthesize comb-shaped cationic poly(2,6-dimethylphenylenoxide) (PPO) polymer anion-exchange membrane (AEM) containing two hexyl side chains.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
192.2 °F - closed cup
Flash Point(C)
89 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Pyrolysis of 1, 1-dihexyl-1-methylamine-2-acylimides.
Wawzonek S and Paschke EE.
The Journal of Organic Chemistry, 36(11), 1474-1476 (1971)
Nanwen Li et al.
Journal of the American Chemical Society, 135(27), 10124-10133 (2013-06-01)
To produce an anion-conductive and durable polymer electrolyte for alkaline fuel cell applications, a series of quaternized poly(2,6-dimethyl phenylene oxide)s containing long alkyl side chains pendant to the nitrogen-centered cation were synthesized using a Menshutkin reaction to form comb-shaped structures.
Erica L Bakota et al.
Rapid communications in mass spectrometry : RCM, 34(5), e8608-e8608 (2019-11-11)
While liquid chromatography/high-resolution mass spectrometry (LC/HRMS) is a versatile analytical technique, it is also sensitive to trace impurities. These impurities may come from a variety of sources, including reagents, solvents, and the sample matrix itself. Impurities in reagents may become
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service