All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H9N3
CAS Number:
Molecular Weight:
159.19
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
124-127 °C (lit.)
SMILES string
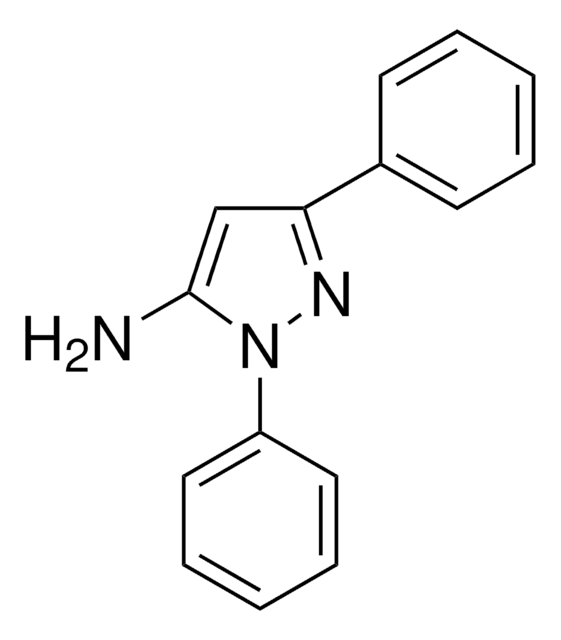
Nc1cc([nH]n1)-c2ccccc2
InChI
1S/C9H9N3/c10-9-6-8(11-12-9)7-4-2-1-3-5-7/h1-6H,(H3,10,11,12)
InChI key
PWSZRRFDVPMZGM-UHFFFAOYSA-N
General description
3-Amino-5-phenylpyrazole (3-phenyl-1H-pyrazol-5-amine), an amino pyrazole derivative, is an aza-heterocyclic amine. It has been reported to be synthesized by heating either 3-amino-4-bromo- or 3-amino-5-phenylisothiazole in the presence of anhydrous hydrazine. On reaction with ZnCl2 it affords chlorido-tris(3-amino-5-phenyl-1Hpyrazole-N2)zinc (II) chloride.
Application
3-Amino-5-phenylpyrazole ((3-phenyl-1H-pyrazol-5-amine) may be used in the synthesis of the following:
- Urea derivatives by reaction with azido(6-(benzofuran-2-yl)-2-methylpyridin-3-yl) methanone.
- 2-Mercaptoacetamide analogs by treating with thioglycolic acid.
- 3-(Substituentpyrimidayl)-5,6-benzocoumarins by treating with 3-(2′-formyl-1′-chlorovinyl)-5,6-benzocoumarin.
- Substituted 2,7-diphenylpyrazolo[1,5-a]pyrimidine-5-carboxylic esters by reacting with substituted β-diketo esters.
- N-ethoxycarbonylthiourea derivative by reacting with ethoxycarbonyl isothiocyanate.
- Heterobiaryl pyrazolo[3,4-b]pyridines by reacting with indole-3-carboxaldehyde.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Crystal structure of chlorido-tris (3-amino-5-phenyl-1H pyrazole-N2) zinc (II) chloride, [ZnCl (C9H9N3)3] Cl.
Jacimovic ZK, et al
Zeitschrift fur Kristallographie, 226(3), 397-399 (2011)
Scott T Moe et al.
Bioorganic & medicinal chemistry, 17(8), 3072-3079 (2009-03-31)
Botulinum neurotoxin elicits its paralytic activity through a zinc-dependant metalloprotease that cleaves proteins involved in neurotransmitter release. Currently, no drugs are available to reverse the effects of botulinum intoxication. Herein we report the design of a novel series of mercaptoacetamide
Recent advances in the chemistry of ethoxycarbonyl isothiocyanate and related compounds.
George B and Papadopoulos EP
Journal of Heterocyclic Chemistry, 20(5), 1127-1142 (1983)
Convenient synthesis of some new pyrazolo [5, 1-c] triazines, isoxazolo [3, 4-d] pyrimidine and pyridine derivatives containing benzofuran moiety.
Abdelhamid AO, et al
European Journal of Chemistry, 3(2), 129-137 (2012)
Syntheses of 3-pyrimidyl-and 3-pyranyl-5, 6-benzocoumarin derivatives.
El-Deen IM, et al
Bull. Korean Chem. Soc., 23(4), 610-612 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service