All Photos(1)
About This Item
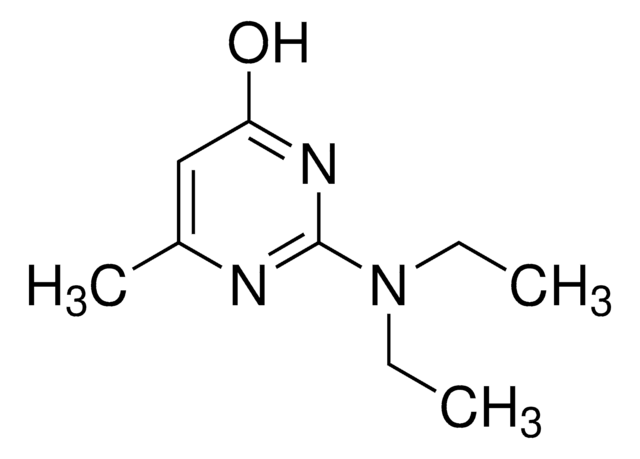
Empirical Formula (Hill Notation):
C6H9N3O
CAS Number:
Molecular Weight:
139.16
Beilstein:
121733
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
mp
156-158 °C (lit.)
solubility
methanol: soluble 1%, clear to very slightly hazy, colorless to yellow
SMILES string
COc1cc(C)nc(N)n1
InChI
1S/C6H9N3O/c1-4-3-5(10-2)9-6(7)8-4/h3H,1-2H3,(H2,7,8,9)
InChI key
SNWZXTZIZWBIDQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Amino-4-methoxy-6-methylpyrimidine (AMMP) is a pyrimidine derivative. It acts as electron donor and forms charge transfer complexes with iodine (σ-electron acceptor). The FTIR and FT-Raman spectra of 2-amino-4-methoxy-6-methylpyrimidine have been recorded to evaluate the energy, structural and thermodynamical parameters. Molecules of AMMP exhibit space group of Pnma and are joined by two N-H…N hydrogen bonds.
Application
2-Amino-4-methoxy-6-methylpyrimidine may be employed as nucleophile to investigate the regioselective introduction of some nucleophiles at the secondary position of Baylis-Hillman adducts. It may be used in the preparation of 2-amino-4-methoxy-6-methylpyrimidin-1-ium picrate.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jerry P Jasinski et al.
Acta crystallographica. Section E, Structure reports online, 66(Pt 5), o1189-o1190 (2010-01-01)
In the title salt, C(6)H(10)N(3)O(+)·C(6)H(2)N(3)O(7) (-), the dihedral angle between the mean planes of the benzene and pyridine rings is 3.1 (1)°. In the cation, the meth-oxy group is almost coplanar with the pyridine ring [C-O-C-N = -0.6 (2)°]. The p-nitro [C-C-N-O
N Prabavathi et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 136 Pt B, 192-204 (2014-12-03)
The FTIR and FT-Raman spectra of 2-amino-4-methoxy-6-methylpyrimidine (AMMP) and 2-amino-5-bromo-6-methyl-4-pyrimidinol (ABMP) have been recorded in the region 4000-450 and 4000-100 cm(-1), respectively. The optimized geometry, frequency and intensity of the vibrational bands of AMMP and ABMP were obtained by the
Facile synthesis of Baylis-Hillman adducts bearing the carbamate or amide functional group at the secondary position.
Lee KY, et al.
Bull. Korean Chem. Soc., 25, 1966-1968 (2004)
Christopher Glidewell et al.
Acta crystallographica. Section C, Crystal structure communications, 59(Pt 1), o9-13 (2002-12-31)
Molecules of 2-amino-4-methoxy-6-methylpyrimidine, C(6)H(9)N(3)O, (I), lie on mirror planes in space group Pnma and are linked by two N-H...N hydrogen bonds [H...N = 2.26 and 2.34 A, N...N = 3.136 (2) and 3.212 (2) A, and N-H...N = 175 and
Usama M Rabie et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 68(3), 605-611 (2007-02-24)
Interactions of some pyrimidine derivatives, 4-amino-2,6-dimethylpyrimidine, kyanmethin, (4AP), 2-amino-4,6-dimethylpyrimidine (2AP), 2-aminopyrimidine (AP), 2-amino-4-methylpyrimidine (AMP), 2-amino-4-methoxy-6-methylpyrimidine (AMMP), and 4-amino-5-chloro-2,6-dimethylpyrimidine (ACDP) as electron donors, with iodine (I(2)), as a typical sigma-electron acceptor, have been studied. Electronic absorption spectra of these interactions in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service