358673
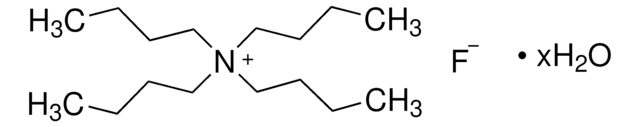
Tetrabutylammonium fluoride on silica gel
~1.5 mmol/g (F-)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3CH2CH2CH2)4N(F)
Molecular Weight:
261.46
Beilstein:
3570522
EC Number:
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
capacity
~1.5 mmol/g (F-)
SMILES string
[F-].CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.FH/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;/h5-16H2,1-4H3;1H/q+1;/p-1
InChI key
FPGGTKZVZWFYPV-UHFFFAOYSA-M
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2
Supplementary Hazards
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mehrdad Balandeh et al.
Journal of the Electrochemical Society, 164(9), G99-G103 (2017-09-12)
Electrochemical fluorination of methyl(phenylthio)acetate was achieved using tetrabutylammonium fluoride (TBAF). Electrochemical fluorination was performed under potentiostatic anodic oxidation using an undivided cell in acetonitrile containing TBAF and triflic acid. The influence of several parameters including: oxidation potential, time, temperature, sonication
Santosh Kumar Behera et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 14(12), 2225-2237 (2015-10-31)
The mechanism for the dual emission of 2-(4'-N,N-dimethylaminophenyl)imidazo[4,5-c]pyridine (DMAPIP-c) in protic solvents was investigated by synthesizing and studying its analogues. Theoretical calculations were carried out to corroborate the experimental findings. The deprotonation studies suggest that the enhancement in the TICT
Delia López-Velázquez et al.
Carbohydrate polymers, 125, 224-231 (2015-04-11)
We have synthesized and characterized five members of a homologous series of side chain polymers of hydroxypropyl cellulose esters obtained by homogeneous esterification with 6-[4'-(ethoxycarbonyl)biphenyl-4-yloxy]hexanoic acid. Two acylation procedures were studied. One procedure involved the acid chloride derivative and the
Eric P Gillis et al.
Journal of medicinal chemistry, 58(21), 8315-8359 (2015-07-23)
The role of fluorine in drug design and development is expanding rapidly as we learn more about the unique properties associated with this unusual element and how to deploy it with greater sophistication. The judicious introduction of fluorine into a
Michelle L Ingalsbe et al.
Bioorganic & medicinal chemistry letters, 19(17), 4984-4987 (2009-08-08)
Due to the increasing number of strains of drug-resistant bacteria, the development of new antibiotics has become increasingly important. The antibacterial properties of quaternary amines and their derivatives on both Gram-positive and Gram-negative bacteria are well known. However, an encompassing
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service