All Photos(1)
About This Item
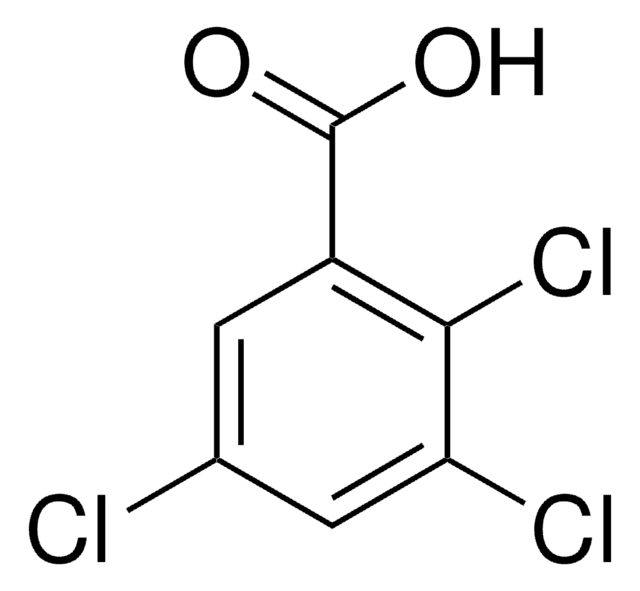
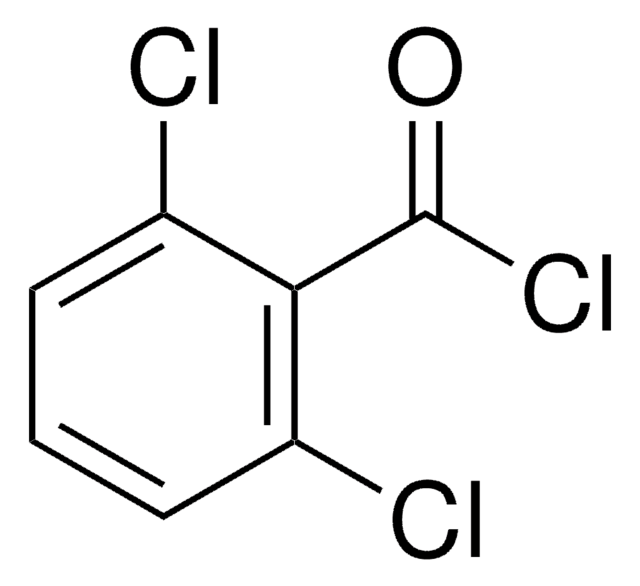
Linear Formula:
Cl3C6H2CO2H
CAS Number:
Molecular Weight:
225.46
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
mp
160-164 °C (lit.)
SMILES string
OC(=O)c1c(Cl)cc(Cl)cc1Cl
InChI
1S/C7H3Cl3O2/c8-3-1-4(9)6(7(11)12)5(10)2-3/h1-2H,(H,11,12)
InChI key
RAFFVQBMVYYTQS-UHFFFAOYSA-N
General description
Structure and hydrogen bonding pattern in 2,4,6-trichlorobenzoic acid is reported.
Application
2,4,6-Trichlorobenzoic acid may be employed as sole carbon and energy supplement for a microbial community. It may be used in the synthesis of (+)-methynolide, the aglycon of a macrolide antibiotic, methymycin.
Reactant involved in:
Cocatalyst for cis-dihydroxylation and epoxidation of alkenes
- Active-sodium-promoted reductive cleavage of halogenated benzoic acids
- Synthesis of aryl aminopyrazole benzamides for use as non-steroidal selective glucocorticoid receptor agonists
- Flame retardant monomer synthesis
- Synthesis of 3,4,7-trisubstituted coumarins for use as antifungals
- Solid-phase synthesis of saphenamycin analogs with antimicrobial activity
Cocatalyst for cis-dihydroxylation and epoxidation of alkenes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
2, 4, 6-Trichlorobenzoic acid: Structure and hydrogen-bonding pattern.
Lalancette RA, et al.
Acta Crystallographica Section C, Structural Chemistry, 52(7), 1801-1804 (1996)
S Moller et al.
Applied and environmental microbiology, 63(6), 2432-2438 (1997-06-01)
A microbial community was cultivated in flow cells with 2,4,6-trichlorobenzoic acid (2,4,6-TCB) as sole carbon and energy source and was examined with scanning confocal laser microscopy and fluorescent molecular probes. The biofilm community which developed under these conditions exhibited a
F R Johannsen et al.
Journal of applied toxicology : JAT, 7(1), 67-70 (1987-02-01)
The acute rat oral LD50 of 2,4,6-Trichlorobenzyl chloride (TCBC) was determined to be 3075 mg/kg. When male and female rats were administered 1500 and 3000 ppm TCBC in the diet for 3 weeks, marked retardation in weight gain was observed.
Hong-Se Oh et al.
Organic & biomolecular chemistry, 7(21), 4458-4463 (2009-10-16)
Methynolide and 10-epi-methynolide were synthesized from the necessary segments, which were prepared by the addition of Grignard reagents to the corresponding alpha-alkoxyketones utilizing 1,2-stereochemical selection based on Cram chelation control. Ring-closing metathesis, as the key reaction, was carried out to
Jin-Fan Zhang et al.
STAR protocols, 3(1), 101071-101071 (2022-01-18)
Fluorescent protein (FP)-based kinase activity biosensors are powerful tools for probing the spatiotemporal dynamics of signaling pathways in living cells. Yet, the limited sensitivity of most kinase biosensors restricts their reliable application in high-throughput detection modalities. Here, we report a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service