218693
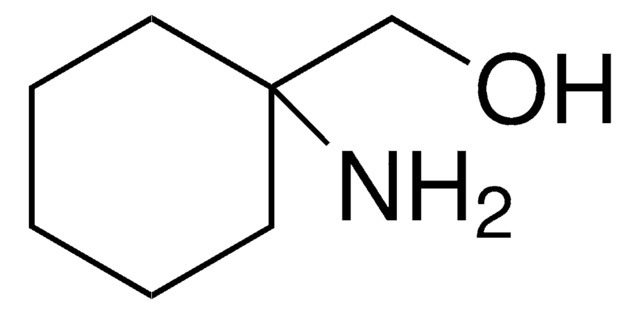
1-Aminocyclohexanecarboxylic acid
98%
Synonym(s):
Homocycloleucine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NC6H10CO2H
CAS Number:
Molecular Weight:
143.18
Beilstein:
2355692
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
reaction suitability
reaction type: solution phase peptide synthesis
mp
>300 °C (lit.)
application(s)
peptide synthesis
SMILES string
NC1(CCCCC1)C(O)=O
InChI
1S/C7H13NO2/c8-7(6(9)10)4-2-1-3-5-7/h1-5,8H2,(H,9,10)
InChI key
WOXWUZCRWJWTRT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Romanelli et al.
Journal of peptide science : an official publication of the European Peptide Society, 7(1), 15-26 (2001-03-14)
Secondary structure formation and stability are essential features in the knowledge of complex folding topology of biomolecules. To better understand the relationships between preferred conformations and functional properties of beta-homo-amino acids, the synthesis and conformational characterization by X-ray diffraction analysis
Wioleta Kowalczyk et al.
Journal of medicinal chemistry, 47(24), 6020-6024 (2004-11-13)
The synthesis and some pharmacological properties of two sets of analogues, one consisting of six peptides with 1-aminocyclohexane-1-carboxylic acid (Acc) in position 2 and the other with the amino acid in position 3, have been described. All the peptides were
Fernando Formaggio et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 8(1), 84-93 (2002-02-02)
The achiral, nitroxyl-containing alpha-amino acid TOAC (TOAC = 2,2,6,6-tetramethylpiperidine-1-oxyl-4-amino-4-carboxylic acid), in combination with the chiral alpha-amino acid C(alpha)-methyl valine [(alphaMe)Val], was used to prepare short peptides (from di- to hexa-) that induced the enantioselective oxidation of racemic 1-phenylethanol to acetophenone.
B L Shulkin et al.
Journal of neurochemistry, 64(3), 1252-1257 (1995-03-01)
The delivery of large neutral amino acids (LNAAs) to brain across the blood-brain barrier (BBB) is mediated by the L-type neutral amino acid transporter present in the membranes of the brain capillary endothelial cell. In experimental animals, the L-system transporter
Olga Labudda-Dawidowska et al.
Journal of medicinal chemistry, 48(25), 8055-8059 (2005-12-13)
In the present work, a sterically constrained noncoded amino acid, 1-aminocyclohexane-1-carboxylic acid (Acc), was substituted in position 8 of the peptide chain of bradykinin (BK) and position 6, 7, or 8 of its B2 receptor antagonist [D-Arg0,Hyp3,Thi,(5,8)D-Phe7]BK, previously synthesized by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service