All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H8N2O3
CAS Number:
Molecular Weight:
204.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
powder
mp
158-160 °C (lit.)
functional group
nitro
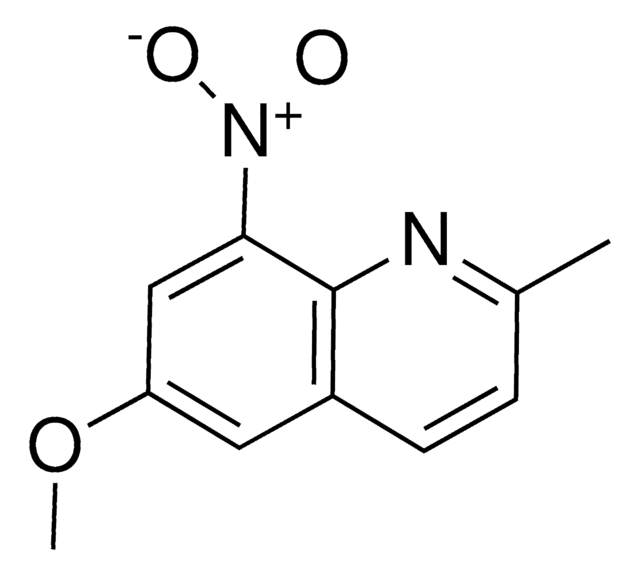
SMILES string
COc1cc([N+]([O-])=O)c2ncccc2c1
InChI
1S/C10H8N2O3/c1-15-8-5-7-3-2-4-11-10(7)9(6-8)12(13)14/h2-6H,1H3
InChI key
MIMUSZHMZBJBPO-UHFFFAOYSA-N
Application
6-Methoxy-8-nitroquinoline was used in the synthesis of:
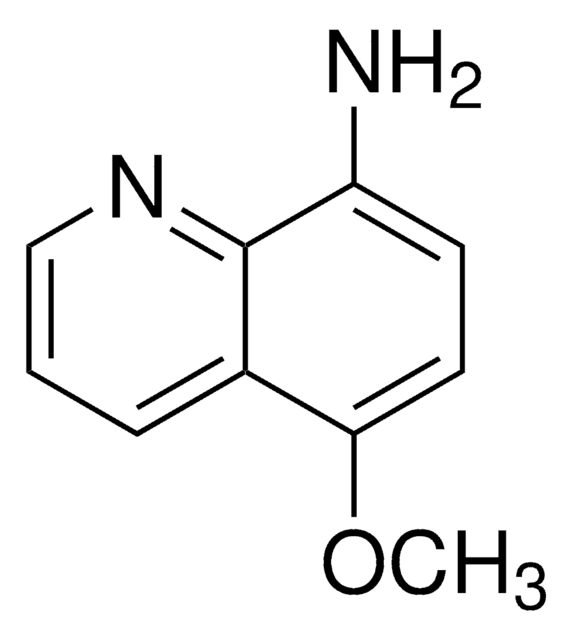
- 6-methoxy-8-aminoquinoline, metabolite of primaquine
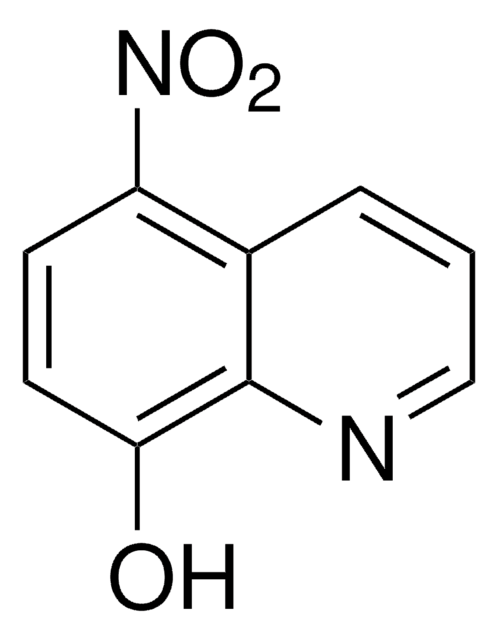
- 6-methoxy-8-hydroxylaminoquinoline
- 2-substituted/2,5-disubstituted-6-methoxy-8-nitroquinolines
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
L J Bolchoz et al.
The Journal of pharmacology and experimental therapeutics, 297(2), 509-515 (2001-04-17)
Primaquine is an important antimalarial agent because of its activity against exoerythrocytic forms of Plasmodium spp. However, methemoglobinemia and hemolytic anemia are dose-limiting side effects of primaquine therapy that limit its efficacy. These hemotoxicities are thought to be mediated by
Suryanarayana Vangapandu et al.
Bioorganic & medicinal chemistry, 12(10), 2501-2508 (2004-04-28)
We report in vitro antimycobacterial properties of ring-substituted quinolines (series 1-4) constituting 56 analogues against drug-sensitive and drug-resistant M. tuberculosis H37Rv strains. The most effective compounds 2h (R1 = R2 = c-C6H11, R3 = NO2, series 1) and 13g (R1
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service