181048
Poly(ethylene-co-acrylic acid)
acrylic acid 20 wt. %, beads
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
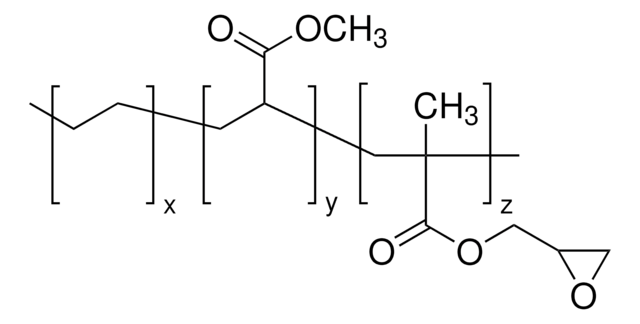
(CH2CH2)x[CH2CH(CO2H)]y
CAS Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
form
beads
composition
acrylic acid, 20 wt. %
density
0.96 g/mL at 25 °C
SMILES string
C=C.OC(=O)C=C
InChI
1S/C3H4O2.C2H4/c1-2-3(4)5;1-2/h2H,1H2,(H,4,5);1-2H2
InChI key
QHZOMAXECYYXGP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Processing and performance additive. Promotes crystallization of PET. Assists dispersion of additives in plastics.
Features and Benefits
Forms reversible ionic clusters (crosslinks). Promotes adhesion to various substrates, tougher, more chemically resistant and more transparent than parent acid copolymer.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
>527.0 °F
Flash Point(C)
> 275 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ning Luo et al.
Colloids and surfaces. B, Biointerfaces, 50(2), 89-96 (2006-06-06)
Poly(ethylene-co-acrylic acid) (EAA) films were reacted with glycine, 12-aminododecanoic acid, aspartic acid, 5-aminoisophthalic acid, ethanolamine, diethylamine, dimethylamine, N-isopropylamine, and dimethylaminoethyleneamine to prepare EAA films with negatively charged, non-charged, hydrophilic, and hydrophobic functionalities. Attenuated total reflectance Fourier transform infrared spectroscopy, differential
Newton C Fawcett et al.
Langmuir : the ACS journal of surfaces and colloids, 20(16), 6651-6657 (2004-07-28)
Yoshimoto et al. [Anal. Chem. 2002, 74, 4306-4309] reported that a quartz crystal microbalance or QCM changed its response to sucrose solutions according to its angle of immersion. The effect was tentatively attributed to gravity-caused stress on the viscous interface
J Wegmann et al.
Analytical chemistry, 73(8), 1814-1820 (2001-05-08)
A new approach for the synthesis of long alkyl chain length stationary phases for use in reversed-phase liquid chromatography is described. Poly(ethylene-co-acrylic acid) copolymers (i.e., (-CH2CH2-)x[CH2CH(CO2H)-]y) with different levels of acrylic acid were covalently bonded to silica via glycidoxypropyl or
A P Martínez-Camacho et al.
Carbohydrate polymers, 91(2), 666-674 (2012-11-06)
The obtaining of chitosan extruded films was possible by using low density polyethylene (LDPE) as a matrix polymer and ethylene-acrylic acid copolymer as an adhesive, in order to ensure adhesion in the interphase of the immiscible polymers. The obtained blend
Optician's occupational allergic contact dermatitis, paresthesia and paronychia caused by anaerobic acrylic sealants.
L Kanerva et al.
Contact dermatitis, 44(2), 117-119 (2001-02-24)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service