V1377
Vinblastine sulfate salt
≥97% (HPLC), powder, plant alkaloid
Synonym(s):
VLB, Vincaleukoblastine sulfate salt
About This Item
Recommended Products
product name
Vinblastine sulfate salt, ≥97% (HPLC)
Quality Level
Assay
≥97% (HPLC)
form
(powder or amorphous or crystalline powder)
color
white to light yellow
mp
267 °C (dec.) (lit.)
absorption
14 at 270 nm in 0.1 M phosphate buffer at 1 mM
16.2 at 259 nm in ethanol at 1 mM
53.7 at 214 nm in ethanol at 1 mM
antibiotic activity spectrum
neoplastics
Mode of action
DNA synthesis | interferes
originator
Eli Lilly
storage temp.
2-8°C
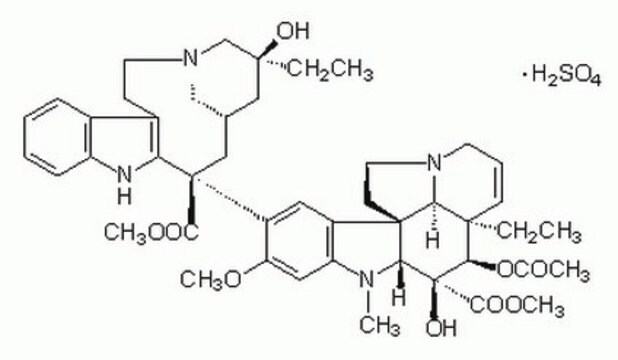
SMILES string
OS(O)(=O)=O.[H][C@@]12CN(CCc3c([nH]c4ccccc34)[C@@](C1)(C(=O)OC)c5cc6c(cc5OC)N(C)[C@@]7([H])[C@](O)([C@H](OC(C)=O)[C@]8(CC)C=CCN9CC[C@]67[C@]89[H])C(=O)OC)C[C@](O)(CC)C2
InChI
1S/C46H58N4O9.H2O4S/c1-8-42(54)23-28-24-45(40(52)57-6,36-30(15-19-49(25-28)26-42)29-13-10-11-14-33(29)47-36)32-21-31-34(22-35(32)56-5)48(4)38-44(31)17-20-50-18-12-16-43(9-2,37(44)50)39(59-27(3)51)46(38,55)41(53)58-7;1-5(2,3)4/h10-14,16,21-22,28,37-39,47,54-55H,8-9,15,17-20,23-26H2,1-7H3;(H2,1,2,3,4)/t28-,37-,38+,39+,42-,43+,44+,45-,46-;/m0./s1
InChI key
KDQAABAKXDWYSZ-PNYVAJAMSA-N
Gene Information
human ... TBCC(6903) , TUBA1A(7846) , TUBA1B(10376) , TUBA1C(84790) , TUBA3C(7278) , TUBA3E(112714) , TUBA4A(7277) , TUBB(203068) , TUBB1(81027) , TUBB2A(7280) , TUBB2B(347733) , TUBB3(10381) , TUBB4A(10382) , TUBB4B(10383) , TUBB6(84617) , TUBB8(347688)
Looking for similar products? Visit Product Comparison Guide
Application
- as a microtubule depolymerizing drug for the synchronization of human cell lines in G2/M phase
- as a multidrug resistance screening substrate in human colon cancer cell line (HCT116) cell line
- as an antimicrotubule agent in sub perineural glia of Drosophila brain
Biochem/physiol Actions
Features and Benefits
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Muta. 2 - Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Discover Bioactive Small Molecules for ADME/Tox
Discover Bioactive Small Molecules for ADME/Tox
Discover Bioactive Small Molecules for ADME/Tox
Discover Bioactive Small Molecules for ADME/Tox
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service