E0885
Enterokinase from porcine intestine
lyophilized powder, ≥100 units/mg protein
Synonym(s):
Enteropeptidase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
form
lyophilized powder
Quality Level
specific activity
≥100 units/mg protein
mol wt
150 kDa
purified by
chromatography
composition
Protein, ≥20% Lowry
foreign activity
aminopeptidase ≤1.5%
trypsin ≤1%, free
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Application
Enterokinase from porcine intestine has been used in a study to investigate complementary DNA cloning and sequencing of rat enteropeptidase. Enterokinase from porcine intestine has also been used to learn more about the insulinotropic region of the gastric inhibitory polypeptide.
The enzyme from Sigma has been used to activate zymogens in order to detect trypsin activity. The study to investigated the structural and evolutionary consequences of unpaired cysteines in trypsinogen. The product has been used to measure trypsin while studying the effect of pesticide induced alterations in gene expression in the lobster, Homarus americanus The enzyme from Sigma has been used to develop a novel assay for measuring levels of lipid-free apoA-I in the presence of lipid-bound apoA-I. Enteropeptidase can specifically cleave human lipid-free apoA-I but not its lipid-bound form resulting in an N-terminal fragment of 22 kDa. It has also been used in a study to examine the effect of calcium and phytic acid on the activation of trypsinogen and the stability of trypsin.
Biochem/physiol Actions
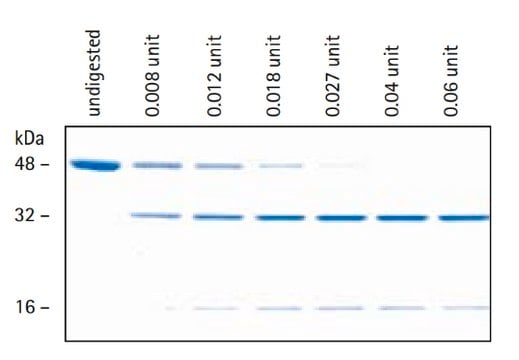
Enterokinase is a membrane bound serine protease that specifically and rapidly converts trypsinogen to trypsin, thereby, triggering the conversion of other zymogens to active enzymes. It has a molecular mass of approximately 150 kDa. The enzyme is a heterodimer consisting of 35-47 kDa subunits. The light and the heavy chains are linked by two disulfide bridges. It is a glycoprotein containing 35% carbohydrate. The polypeptide chain of trypsinogen is hydrolyzed only after an -(Asp)4-Lys- sequence. The enzyme is inhibited by soybean trypsin inhibitor. Enterokinase is typically used in protein modification and amino acid sequence determination.
Unit Definition
One unit will produce 1.0 nanomole of trypsin from trypsinogen per min at pH 5.6 at 25 °C.
Physical form
Lyophilized powder containing sodium phosphate buffer salts
substrate
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Michael N Horst et al.
Comparative biochemistry and physiology. Part D, Genomics & proteomics, 2(1), 44-52 (2007-03-01)
Using subtractive hybridization, we have identified 17 genes that are either up- or down-regulated in the hepatopancreas (Hp) of the lobster, Homarus americanus, by acute exposure to the juvenile hormone analog methoprene. The expression of some of the genes obtained
Effect of calcium and phytic acid on the activation of trypsinogen and the stability of trypsin.
Caldwell RA
Journal of Agricultural and Food Chemistry, 40 (1), 43-46 (1992)
W Safi et al.
Journal of lipid research, 42(5), 864-872 (2001-05-16)
Recent studies indicate that certain lipid-poor forms of apolipoprotein (apo)A-I may be particularly important in promoting cholesterol release from overburdened cells in the periphery. However, a detailed understanding of the physiological relevance of these species has been hampered by the
Erzsébet Kénesi et al.
Biochemical and biophysical research communications, 309(4), 749-754 (2003-09-19)
Vertebrate trypsins usually contain six disulfide bonds but human trypsin 1 (PRSS1) contains only five and human trypsin 2 (PRSS2) contains only four. To elucidate possible evolutionary pathways leading to the loss of disulfide bonds, we have constructed mutants lacking
Complementary DNA cloning and sequencing of rat enteropeptidase and tissue distribution of its mRNA.
N Yahagi et al.
Biochemical and biophysical research communications, 219(3), 806-812 (1996-02-27)
A cDNA clone encoding enteropeptidase (EC 3.4.21.9), a key enzyme for the conversion of trypsinogen to trypsin, was isolated from a rat duodenal mucosa cDNA library. Sequences of the 3585 base pair clone predicted that enteropeptidase is synthesized as a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service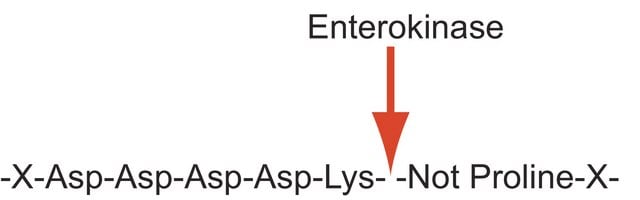



![4-(N-[2,4-Diamino-6-pteridinylmethyl]amino)benzoic acid sodium salt ≥95% (TLC)](/deepweb/assets/sigmaaldrich/product/structures/855/619/06a9574b-3e45-48aa-85c1-2f25917fa5a7/640/06a9574b-3e45-48aa-85c1-2f25917fa5a7.png)