ABT1387
Anti-Phospho-Lamin A/C (Ser404)
from rabbit
Synonym(s):
Prelamin A/C, Renal carcinoma antigen NY-REN-32
About This Item
Recommended Products
biological source
rabbit
antibody form
affinity isolated antibody
antibody product type
primary antibodies
clone
polyclonal
species reactivity
human
technique(s)
immunofluorescence: suitable
inhibition assay: suitable (peptide)
western blot: suitable
isotype
IgG
NCBI accession no.
UniProt accession no.
target post-translational modification
phosphorylation (pSer404)
Gene Information
human ... LMNA(4000)
General description
Is cleaved to generate Lamin A/C. Farnesylation of prelamin-A/C facilitates nuclear envelope targeting and subsequent cleavage by ZMPSTE24/FACE1 to remove the farnesyl group produces mature Lamin-A/C that is inserted into the nuclear lamina. Lamin A and C are present in equal amounts in the lamina of mammals and they play an important role in nuclear assembly, chromatin organization, nuclear membrane and telomere dynamics. Lamins are shown to be essential for normal development of peripheral nervous system and skeletal muscle and for muscle satellite cell proliferation. Lamins also prevent fat infiltration of muscle and bone marrow, helping to maintain the volume and strength of skeletal muscle and bone. Phosphorylation of Lamins is reported to occur continuously throughout all interphase periods and takes place mainly on the assembled lamina. Phosphorylation of the major polypeptides of the lamina induces laminar disassembly during mitosis. Phosphorylated Lamin-A/C localizes to nucleoplasm. Lamin A/C undergoes phosphorylation at multiple sites and one of the best characterized phosphorylation sites is on Serine 22 and it is phosphorylated during interphase. Phosphorylation of Serine 22 stabilizes Lamin A/C. Overexpression of Lamin-A is shown to result in greater phosphorylation of Serine 22 and 390 and Lamin A/C knockdowns display reduced phosphorylation at both sites, which helps in maintaining the integrity of the diminished lamina. Lamin A/C can undergoes phosphorylation on Serine 404 by Akt1 and Ser4040 phosphorylated Lamin undergoes rapid lysosomal degradation. Mutations in LMNA gene can cause Emery-Dreifuss muscular dystrophy 2 and 3, which are characterized by weakness and atrophy of muscle without involvement of the nervous system and cardiac conduction defects. Some mutations have also been linked to familial Lipodystrophy that leads to the loss of subcutaneous adipose tissue in the lower parts of the body and accumulation of adipose tissue in the face and neck. (Ref.: Buxboim, A., et al. (2014). Curr. Biol. 24(16): 1909-1917; Toker, A., and Marmiroli, S. (2014). Adv. Biol. Regul. 55: 28-38).
Specificity
Immunogen
Application
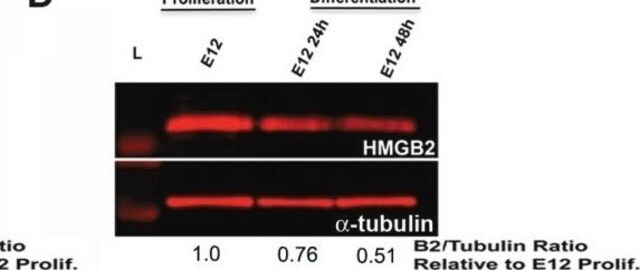
Peptide Inhibition Analysis: A 1:500 dilution from a representative lot was used with A549 cells (specific for Lamin A/C phosphorylation) for peptide block analysis.
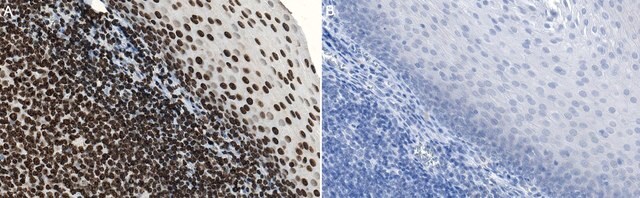
Cell Structure
Quality
Western Blotting Analysis: A 1:500 dilution of this antibody detected Phospho-Lamin A/C (Ser404) in A549 cells (specific for Lamin A/C phosphorylation).
Target description
Physical form
Storage and Stability
Other Notes
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service