T35505
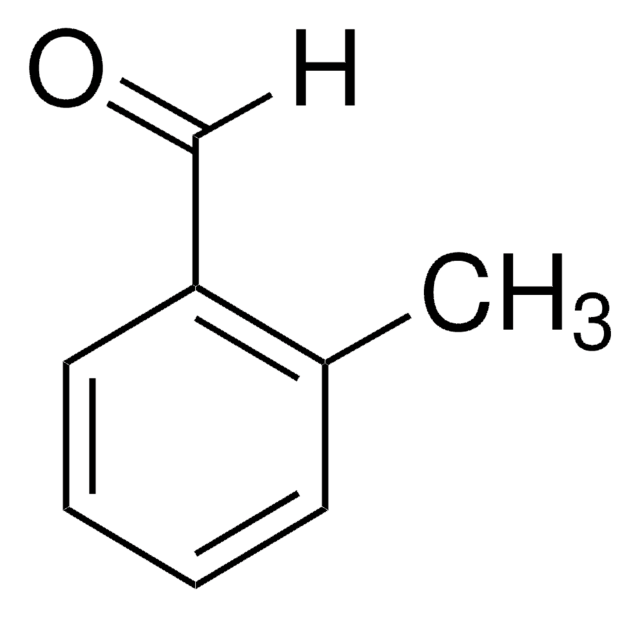
m-Tolualdehyde
97%
Synonym(s):
3-Methylbenzaldehyde
About This Item
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.541 (lit.)
bp
199 °C (lit.)
80-82 °C/11 mmHg (lit.)
density
1.021 g/mL at 20 °C
1.019 g/mL at 25 °C (lit.)
SMILES string
[H]C(=O)c1cccc(C)c1
InChI
1S/C8H8O/c1-7-3-2-4-8(5-7)6-9/h2-6H,1H3
InChI key
OVWYEQOVUDKZNU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
181.4 °F - closed cup
Flash Point(C)
83 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
-Tolualdehyde; Valeraldehyde; Isovaleraldehyde
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service