P73404
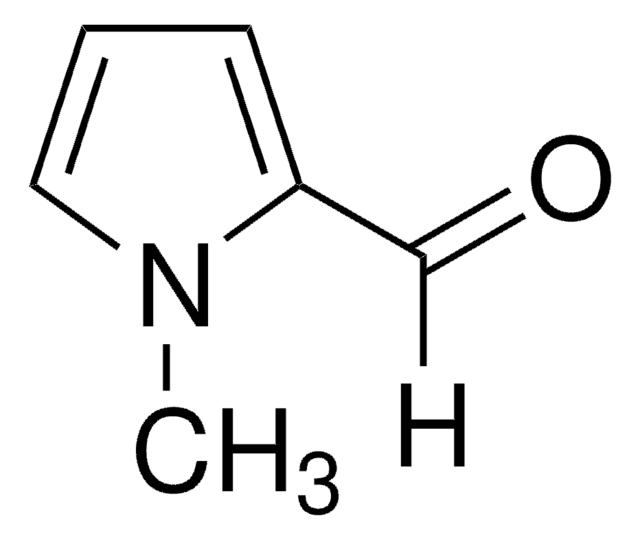
Pyrrole-2-carboxaldehyde
98%
Synonym(s):
2-Formylpyrrole
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H5NO
CAS Number:
Molecular Weight:
95.10
Beilstein:
105745
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
crystals
bp
217-219 °C (lit.)
mp
43-46 °C (lit.)
storage temp.
2-8°C
SMILES string
[H]C(=O)c1ccc[nH]1
InChI
1S/C5H5NO/c7-4-5-2-1-3-6-5/h1-4,6H
InChI key
ZSKGQVFRTSEPJT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Pyrrole-2-carboxaldehydesis a heterocyclic building blocks characterized by a pyrrole ring with a formylgroup attached at the 2-position used in the production of various biologicallyactive compounds. Highly functionalized pyrrole-2-carboxaldehydes have beenutilized as an intermediate in the creation of oligopyrrole macrocycles.
Application
- Pyrimidine-based functional fluorescent organic nanoparticle probe for detection of Pseudomonas aeruginosa.: This study used pyrrole-2-carboxaldehyde to develop a fluorescent nanoparticle probe based on pyrimidine for detecting Pseudomonas aeruginosa, enhancing diagnostic capabilities in microbiology (Kaur G et al., 2015).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
224.6 °F - closed cup
Flash Point(C)
107 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Michiko Kimoto et al.
Nucleic acids research, 35(16), 5360-5369 (2007-08-19)
Fluorescent labeling of nucleic acids is widely used in basic research and medical applications. We describe the efficient site-specific incorporation of a fluorescent base analog, 2-amino-6-(2-thienyl)purine (s), into RNA by transcription mediated by an unnatural base pair between s and
Takumi Ishizuka et al.
Chemical communications (Cambridge, England), 48(88), 10835-10837 (2012-10-04)
Toward new biotechnology by genetic alphabet expansion, we developed an efficient site-specific labeling method for large RNA molecules. The combination of unnatural base pair transcription and post-transcriptional modification by click chemistry enables simple RNA labeling with a wide variety of
Tsuneo Mitsui et al.
Journal of the American Chemical Society, 125(18), 5298-5307 (2003-05-02)
An unnatural hydrophobic base, pyrrole-2-carbaldehyde (denoted as Pa), was developed as a specific pairing partner of 9-methylimidazo[(4,5)-b]pyridine (Q). The Q base is known to pair with 2,4-difluorotoluene (F) as an isostere of the A-T pair, and F also pairs with
Corey A Rice et al.
The Journal of chemical physics, 126(13), 134313-134313 (2007-04-14)
Intermolecular interactions relevant for antiparallel beta-sheet formation between peptide strands are studied by Fourier transform infrared spectroscopy of the low temperature, vacuum-isolated model compound pyrrole-2-carboxaldehyde and its dimer in the N-H and C=O stretching range. Comparison to quantum chemical predictions
Yasushi Hikida et al.
Nature protocols, 5(7), 1312-1323 (2010-07-03)
Methods for fluorescent probing at a defined position of RNA provide powerful tools for analyzing the local structural conformation of functional RNA molecules by tracking fluorescence changes. In this article, we describe the site-specific fluorescent probing of RNA by transcription
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service