All Photos(1)
About This Item
Empirical Formula (Hill Notation):
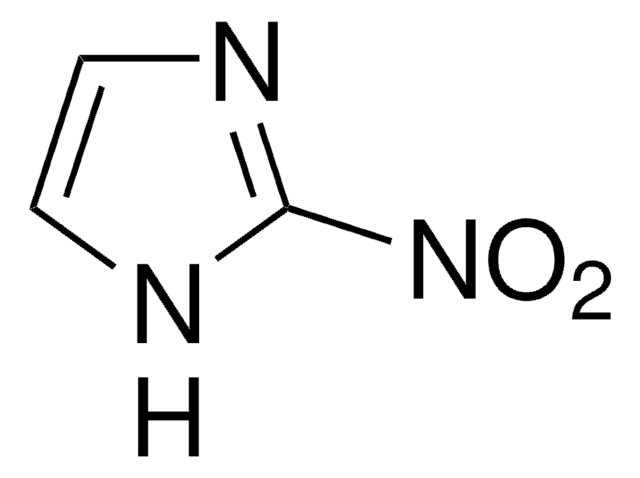
C4H5N3O2
CAS Number:
Molecular Weight:
127.10
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
249 °C (lit.)
functional group
nitro
SMILES string
Cc1[nH]cnc1[N+]([O-])=O
InChI
1S/C4H5N3O2/c1-3-4(7(8)9)6-2-5-3/h2H,1H3,(H,5,6)
InChI key
WSYOWIMKNNMEMZ-UHFFFAOYSA-N
General description
5-Methyl-4-nitroimidazole, an imidazole derivative, is a heterocyclic NH-acid. Its nitration in the presence of acetic anhydride/glacial acetic acid has been studied.
Application
5-Methyl-4-nitroimidazole may be used in the following:
- To trap the reactive 1:1 intermediate formed during the reaction between triphenylphosphine and dibenzoylacetylene.
- As a starting reagent in the synthesis of pyrazole derivatives.
- Fabrication of zeolitic imidazolate frameworks (ZIFs).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Reaction between heterocyclic NH-acids and dibenzoylacetylene in the presence of triphenylphosphine. Simple synthesis of 1-(3-furyl)-1H-imidazole derivatives.
Yavari I, et al.
Tetrahedron Letters, 43(51), 9449-9452 (2002)
Synthesis, metabolism and structure-mutagenicity relationships of novel 4-nitro-(imidazoles and pyrazoles) in Salmonella typhimurium.
Hrelia P, et al.
Mutation Research. Fundamental and Molecular Mechanisms of Mutagenesis, 397(2), 293-301 (1998)
1, 4-Dinitroimidazole and derivatives. Structure and thermal rearrangement.
Grimmett MR, et al.
Australian Journal of Chemistry, 42(8), 1281-1289 (1989)
Localized cell stimulation by nitric oxide using a photoactive porous coordination polymer platform.
Stéphane Diring et al.
Nature communications, 4, 2684-2684 (2013-10-26)
Functional cellular substrates for localized cell stimulation by small molecules provide an opportunity to control and monitor cell signalling networks chemically in time and space. However, despite improvements in the controlled delivery of bioactive compounds, the precise localization of gaseous
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service