127574
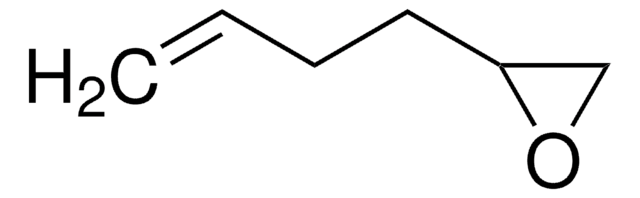
3,4-Epoxy-1-butene
98%
Synonym(s):
2-Vinyloxirane, 3,4-Epoxy-1-butene, Butadiene monoxide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H6O
CAS Number:
Molecular Weight:
70.09
Beilstein:
103170
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.417 (lit.)
bp
65-66 °C (lit.)
density
0.87 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C=CC1CO1
InChI
1S/C4H6O/c1-2-4-3-5-4/h2,4H,1,3H2
InChI key
GXBYFVGCMPJVJX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-58.0 °F - closed cup
Flash Point(C)
-50 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Valeria Di Bussolo et al.
The Journal of organic chemistry, 69(21), 7383-7386 (2004-10-09)
The reaction of alpha vinyl oxirane 5, prepared through a new route to the d-gulal system, with O-nucleophiles (alcohols and di-O-isopropylidene-alpha-d-monosaccharides) and C-nucleophiles (lithium alkyls) affords, in a completely stereoselective way, the corresponding 2-unsaturated alpha O- and C-glycosides having the
Lindsay A Batory et al.
Journal of the American Chemical Society, 128(50), 16054-16055 (2006-12-15)
A novel copper-catalyzed vinyl oxirane ring expansion protocol has been developed. A wide range of vinyl oxiranes can be rearranged to 2,5-dihydrofurans in excellent yields in the presence of electrophilic copper(II) acetylacetonate catalysts. Regioisomeric vinyl oxiranes can be converted to
Charlotta Fred et al.
Mutation research, 585(1-2), 21-32 (2005-06-01)
1,3-Butadiene and isoprene (2-methyl-1,3-butadiene) are chemically related substances that are carcinogenic to rodents. The overall aim of this work is to elucidate the role of the genotoxic action of diepoxide metabolites in the carcinogenesis of the dialkenes. In vivo doses
Quanxin Meng et al.
Chemico-biological interactions, 166(1-3), 207-218 (2006-07-21)
The carcinogenicity of 1,3-butadiene (BD) is related to its bioactivation to several DNA-reactive metabolites; accumulating evidence suggests that the stereochemistry of these BD intermediates may play a significant role in the mutagenic and carcinogenic actions of the parent compound. The
Ewan D Booth et al.
Chemico-biological interactions, 147(2), 195-211 (2004-03-12)
Male Sprague-Dawley rats and B6C3F1 mice were exposed to either a single 6h or a multiple (5) daily (6h) nose-only dose of 1,3-[2,3-(14)C]-butadiene at exposure concentrations of nominally 1, 5 or 20 ppm. The aim was to compare the results
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service