E6412
Cellobiohydrolase I from Hypocrea jecorina
0.13 U/mg, recombinant, expressed in corn
Synonym(s):
Cel7A, Cellobiosidase, Cellulase
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Recommended Products
recombinant
expressed in corn
Quality Level
form
liquid
specific activity
0.13 U/mg
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
greener alternative category
shipped in
dry ice
storage temp.
−20°C
Related Categories
General description
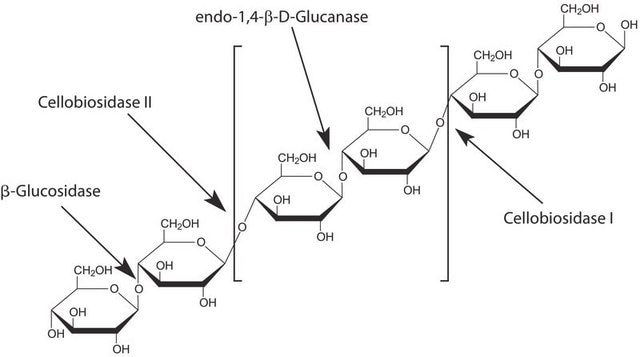
Cellubiohydrolase I is an enzyme present in many fungi, but particularly wood rot fungi. It is a monomer of 53 kDa with a catalytic domain and a cellulose binding domain. The reaction adds water to the glucose bonds in cellulose (non-reducing ends of the chain), yielding cellobiose.
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Find details here.
Application
Cellobiohydrolase I can be used in combination with endocellulases and b-glucosidase to produce glucose from cellulose.
Biochem/physiol Actions
Cellobiohydrolase (CBH) is a cellulase which degrades cellulose by hydrolysing the 1,4-β-D-glycosidic bonds. CBH is an exocellulase which cleaves two to four units from the ends of cellulose. CBH I cleaves progressively from the reducing end. CBH I is commonly used in detergents for cleaning textiles. Its ezymatic activity ranges from 37° C to 50° C, with its optimal temperature being approximately 45° C. The optimum pH for the enzyme is 5-6.
Unit Definition
Unit Definition: A unit will turn over 1 nmole of methyl-umbelliferyl beta-D cellobioside per min at pH 5 at 50° C.
Physical form
Provided as an ammonium sulfate precipitate with the source as recombinant maize.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jenni Liisa Rahikainen et al.
Bioresource technology, 146, 118-125 (2013-08-08)
Non-productive enzyme adsorption onto lignin inhibits enzymatic hydrolysis of lignocellulosic biomass. Three cellobiohydrolases, Trichoderma reesei Cel7A (TrCel7A) and two engineered fusion enzymes, with distinctive modular structures and temperature stabilities were employed to study the effect of temperature on inhibition arising
Nicolaj Cruys-Bagger et al.
The Journal of biological chemistry, 287(22), 18451-18458 (2012-04-12)
The transient kinetic behavior of enzyme reactions prior to the establishment of steady state is a major source of mechanistic information, yet this approach has not been utilized for cellulases acting on their natural substrate, insoluble cellulose. Here, we elucidate
Daisuke Taneda et al.
Bioresource technology, 121, 154-160 (2012-08-04)
The effect of enzyme loading under static and agitated conditions was investigated. Enzymatic hydrolysis of 10 w/v% de-lignified cellulose slurry such as filter paper, avicel and pulp was conducted under agitated (120 rpm) and static condition, and the enzyme loading
K E Eriksson et al.
European journal of biochemistry, 51(1), 213-218 (1975-02-03)
An exo-1,4-beta-glucanase from culture solution of the rot fungus Sporotrichum pulverulentum (formerly called Chrysosporium lignorum) grown on powder cellulose as the sole carbon source has been extensively purified and characterized with respect to some physico-chemical properties. The purification has been
Barry Z Shang et al.
The Journal of biological chemistry, 288(40), 29081-29089 (2013-08-21)
Interprotein and enzyme-substrate couplings in interfacial biocatalysis induce spatial correlations beyond the capabilities of classical mass-action principles in modeling reaction kinetics. To understand the impact of spatial constraints on enzyme kinetics, we developed a computational scheme to simulate the reaction
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service