DUO82064
Duolink® In Situ Microplate Nuclear Stain, Anti-Fade
Synonym(s):
in situ Proximity Ligation Assay reagent, Protein Protein Interaction Assay reagent
About This Item
Recommended Products
product line
Duolink®
technique(s)
proximity ligation assay: suitable
fluorescence
λex 360 nm; λem 460 nm
suitability
suitable for fluorescence-detection automated sequencing
suitable for microtiter plates
shipped in
dry ice
storage temp.
−20°C
Application
Use the Multiwell Plates modifications to the Duolink® In Situ Fluorescence Protocol to run an experiment with this product. A set of short instructions can also be used.
Visit our Duolink® PLA Resource Center for information on how to run a Duolink® experiment, applications, troubleshooting, and more.
To perform a complete Duolink® PLA in situ experiment you will need two primary antibodies (PLA, IHC, ICC or IF validated) that recognize two target epitopes. Other necessary reagents include a pair of PLA probes from different species (one PLUS and one MINUS), detection reagents, wash buffers, and mounting medium. Note that the primary antibodies must come from the same species as the Duolink® PLA probes. Analysis is carried out using standard immunofluorescence assay equipment.
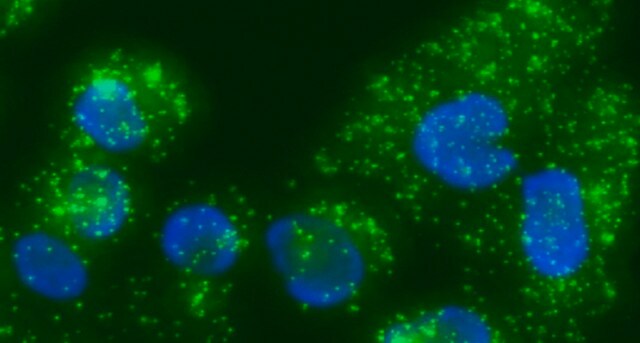
Duolink® In Situ Microplate Nuclear Stain and Anti-Fade are intended to be used after staining cells with Duolink® In Situ in microtiter plates. See the datasheet for more information.
Application Note
Two primary antibodies raised in different species are needed. Test your primary antibodies (IgG-class, mono- or polyclonal) in a standard immunofluorescence (IF), immunohistochemistry (IHC) or immunocytochemistry (ICC) assay to determine the optimal fixation, blocking, and titer conditions. Duolink® in situ reagents are suitable for use on fixed cells, cytospin cells, cells grown on slide, formalin-fixed, paraffin embedded (FFPE), or tissue (fresh or frozen). No minimum number of cells is required.
Let us do the work for you, learn more about our Custom Service Program to accelerate your Duolink® projects
View full Duolink® product list
Features and Benefits
- No overexpression or genetic manipulation required
- High specificity (fewer false positives)
- Single molecule sensitivity due to rolling circle amplification
- Relative quantification possible
- No special equipment needed
- Quicker and simpler than FRET
- Increased accuracy compared to co-IP
- Publication-ready results
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Skin Sens. 1
Storage Class Code
12 - Non Combustible Liquids
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service