79470
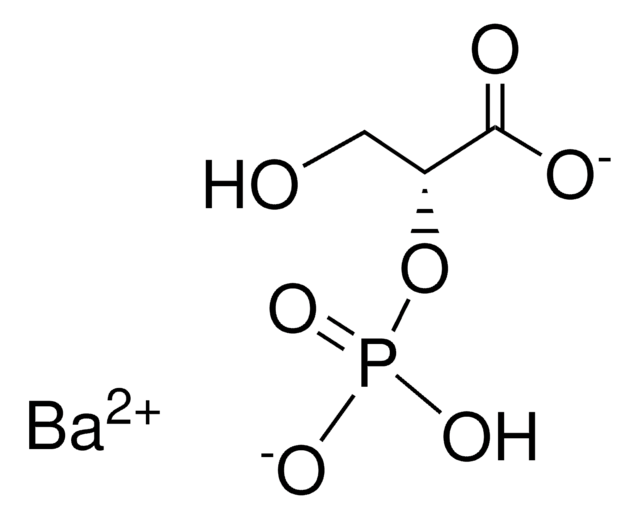
D(+)-2-Phosphoglyceric acid sodium salt hydrate
≥75% (calc. on dry substance, enzymatic)
Synonym(s):
D-Glycerate 2-phosphate sodium salt, Sodium D-2-phosphoglycerate hydrate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C3H7O7P · xNa+ · yH2O
CAS Number:
Molecular Weight:
186.06 (anhydrous free acid basis)
UNSPSC Code:
12352204
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Assay
≥75% (calc. on dry substance, enzymatic)
form
powder
impurities
≤1% Pi
≤35% water
storage temp.
−20°C
SMILES string
O=C(O)[C@@H](CO)OP(O)(O)=O.C.C
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
The enzyme enolase is responsible for reversible conversion of 2-phosphoglycerate (2-PG) into phosphoenolpyruvate (PEP). Phosphoglycerate mutase causes reversible isomerization of 3-phosphoglycerate and 2-phosphoglycerate in glycolysis and gluconeogenesis.
Enantiomerically pure metabolite of glycolysis/gluconeogenesis and also substrate for a number of important enzymes in central metabolism like enolase and phosphoglycerate mutase.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T M Larsen et al.
Biochemistry, 35(14), 4349-4358 (1996-04-09)
The equilibrium mixture of yeast enolase with substrate, 2-phospho-D-glycerate (2-PGA), and product, phosphoenolpyruvate (P-enolpyruvate), has been crystallized from solutions of poly(ethylene glycol) (PEG) at pH 8.0. Crystals belong to the space group C2 and have unit cell dimensions a =
Molecular characterization of phosphoglycerate mutase in archaea.
van der Oost J, et al.
FEMS Microbiology Letters, 212, 111-120 (2002)
Cloning, expression and characterization of an extracellular enolase from Leuconostoc mesenteroides.
Jin-Ha Lee et al.
FEMS microbiology letters, 259(2), 240-248 (2006-06-01)
Enolase on the surface of streptococci putatively facilitates pathogenic invasion of the host organisms. The related Leuconostoc mesenteroides 512FMCM is nonpathogenic, but it too has an extracellular enolase. Purified isolates of extracellular dextransucrase from cultures of L. mesenteroides contain minute
Grégory Boël et al.
Journal of molecular biology, 337(2), 485-496 (2004-03-09)
We observed that in vivo and in vitro a small fraction of the glycolytic enzyme enolase became covalently modified by its substrate 2-phosphoglycerate (2-PG). In modified Escherichia coli enolase, 2-PG was bound to Lys341, which is located in the active
E Zhang et al.
Biochemistry, 36(41), 12526-12534 (1997-11-05)
Enolase, a glycolytic enzyme that catalyzes the dehydration of 2-phospho-d-glycerate (PGA) to form phosphoenolpyruvate (PEP), is a homodimer in all eukaryotes and many prokaryotes. Here, we report the crystal structure of a complex between yeast enolase and an equilibrium mixture
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service