8.52418
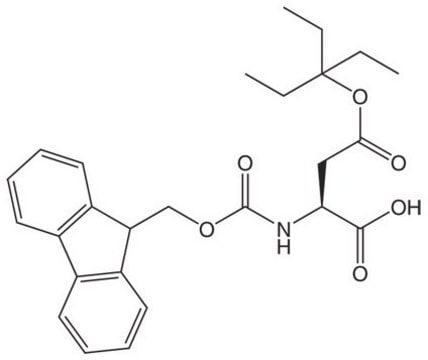
Fmoc-Asp(OBno)-OH
for peptide synthesis, Novabiochem®
Synonym(s):
Fmoc-Asp(OBno)-OH
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C32H43NO6
Molecular Weight:
537.69
UNSPSC Code:
12352209
NACRES:
NA.22
Recommended Products
Product Name
Fmoc-Asp(OBno)-OH, Novabiochem®
Quality Level
product line
Novabiochem®
form
powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
functional group
carboxylic acid
storage temp.
−20°C (−15°C to −25°C)
General description
An excellent derivative for minimizing aspartimide formation during Fmoc SPPS, including those containing the Asp-Gly sequence. The bulky OBno protecting group offers considerably more protection against the formation of aspartimide-related by-products than the commonly used OtBu and OMpe group.,
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Overcoming Aspartimide Formation in Fmoc SPPS
Literature references:
[1] R. Behrendt, et al. (2015) J. Pept. Sci., 21, 680.
[2] R. Behrendt, et al. (2016) J. Pept. Sci., 22, 92.
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Overcoming Aspartimide Formation in Fmoc SPPS
Literature references:
[1] R. Behrendt, et al. (2015) J. Pept. Sci., 21, 680.
[2] R. Behrendt, et al. (2016) J. Pept. Sci., 22, 92.
Application
Recently, Fmoc-Asp(OBno)-OH has been used in the synthesis of the mini-protein Omomyc, which has been shown to repress MYC-dependent gene transcription.,
Analysis Note
Color (visual): white to beige
Appearance of substance (visual): powder, chunks or crystals
Identity (IR): passes test
Purity (TLC (018A)): ≥ 95 %
Enantiomeric purity: ≥ 99.5 % (a/a)
Assay (HPLC, area%): ≥ 97.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
To see the solvent systems used for TLC of Novabiochem® products please click here.
Appearance of substance (visual): powder, chunks or crystals
Identity (IR): passes test
Purity (TLC (018A)): ≥ 95 %
Enantiomeric purity: ≥ 99.5 % (a/a)
Assay (HPLC, area%): ≥ 97.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
To see the solvent systems used for TLC of Novabiochem® products please click here.
Legal Information
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
New t-butyl based aspartate protecting groups preventing aspartimide formation in Fmoc SPPS
R. Behrendt, et al.
Journal of Peptide Science, 21, 680-680 (2015)
Synthesis and evaluation of a multifunctional probe with a high affinity for prostate-specific membrane antigen (PSMA) and bone
Hirata S, et al.
Nuclear Medicine and Biology, 114-115, 34-41 (2022)
Preventing aspartimide formation in Fmoc SPPS of Asp-Gly containing peptides?practical aspects of new trialkylcarbinol based protecting groups
R. Behrendt, et al.
Journal of Peptide Science, 22, 92-92 (2016)
Omomyc Reveals New Mechanisms To Inhibit the MYC Oncogene
Mark J. Demma, et al.
Molecular and Cellular Biology, 39 (2019)
Multiple Synthetic Routes to the Mini-Protein Omomyc and Coiled-Coil Domain Truncations
Brown ZZ, et al.
The Journal of Organic Chemistry, 1466-1466 (2020)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service