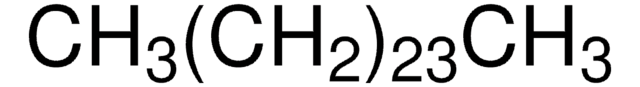All Photos(2)
About This Item
Linear Formula:
CH3(CH2)22CH3
CAS Number:
Molecular Weight:
338.65
Beilstein:
1758462
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
bp
391 °C (lit.)
mp
49-52 °C (lit.)
solubility
chloroform: soluble 10%, clear, colorless
SMILES string
CCCCCCCCCCCCCCCCCCCCCCCC
InChI
1S/C24H50/c1-3-5-7-9-11-13-15-17-19-21-23-24-22-20-18-16-14-12-10-8-6-4-2/h3-24H2,1-2H3
InChI key
POOSGDOYLQNASK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Tetracosane is a linear alkane. Structural characteristics of confined squalane and tetracosane under shear flow conditions have been investigated. Solubility of tetracosane in ethane and ethylene have been investigated.
Viscosity, liquid density, and surface tension data of its binary and ternary mixtures with various n-alkanes (n-heptane, n-decane or n-hexadecane) have been evaluated. Its solubility in various hydrocarbon solvents (heptane, dodecane, isomeric mixtures of decahydronapthalene, m- and p-xylene) has been studied.
Application
Tetracosane is the suitable solvent used in the synthesis of ZnS nanoparticle. It may be employed as the wax component to study the reduction in pour point of hydrocarbon solvents containing wax crystals on addition of polymer additives.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Solubilities of tetracosane in hydrocarbon solvents.
Brecevic L and Garside J.
Journal of Chemical and Engineering Data, 38(4), 598-601 (1993)
Viscosity and liquid density of asymmetric hydrocarbon mixtures.
Queimada AJ, et al.
International Journal of Thermophysics, 24(5), 1221-1239 (2003)
Shear behavior of squalane and tetracosane under extreme confinement. I. Model, simulation method, and interfacial slip.
Gupta SA, et al.
J. Chem. Phys. , 107(23), 10316-10326 (1997)
Pan Liao et al.
Nature chemical biology, 17(2), 138-145 (2020-10-21)
The plant cuticle is the final barrier for volatile organic compounds (VOCs) to cross for release to the atmosphere, yet its role in the emission process is poorly understood. Here, using a combination of reverse-genetic and chemical approaches, we demonstrate
Veronica Lolli et al.
Foods (Basel, Switzerland), 9(7) (2020-07-12)
Cyclopropenoid fatty acids (CPEFA), found in oilseeds from Malvaceae and Sterculiaceae, have been shown to interfere with the endogenous synthesis of several bioactive lipids of dairy fat, such as cis-9, trans-11 18:2 and cis-9 18:1, by inhibiting Δ9-desaturase. No previous
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service