B33803
Bicyclo[2.2.1]hepta-2,5-diene
98%
Synonym(s):
2,5-Norbornadiene, NBD
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C7H8
CAS Number:
Molecular Weight:
92.14
Beilstein:
506224
MDL number:
UNSPSC Code:
12352300
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
98%
form
liquid
contains
0.05-0.25% BHT as inhibitor
refractive index
n20/D 1.470 (lit.)
bp
89 °C (lit.)
density
0.906 g/mL at 25 °C (lit.)
SMILES string
C1[C@H]2C=C[C@@H]1C=C2
InChI
1S/C7H8/c1-2-7-4-3-6(1)5-7/h1-4,6-7H,5H2/t6-,7+
InChI key
SJYNFBVQFBRSIB-KNVOCYPGSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Intermediate in prostaglandin synthesis.
Features and Benefits
Intermediate in prostaglandin synthesis.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
-5.8 °F - closed cup
Flash Point(C)
-21 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M C Whalen et al.
Canadian journal of botany. Journal canadien de botanique, 66(4), 719-723 (1988-04-01)
The control of primary root growth in Zea mays cv. Merit by ethylene was examined. At applied concentrations of ethylene equal to or greater than 0.1 microliter L-1, root elongation during 24 h was inhibited. The half-maximal response occurred at
Francesco Babudri et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 6(4), 361-364 (2007-04-04)
A conjugated alternating copolymer containing norbornadiene and bis(ethynylene)phenylene units was prepared by the Cassar-Heck-Sonogashira cross-coupling reaction. Its electroluminescence was tested in a device, and its fluorescence colour could be tuned by light-induced norbornadiene-quadricyclane isomerization.
Zhiru Ma et al.
Magnetic resonance in chemistry : MRC, 45(5), 393-400 (2007-03-31)
This paper presents novel measurements and calculations of the olefinic (13)C chemical shift tensor principal values in several metal diene complexes. The experimental values and the calculations show shifts as large as 70 ppm with respect to the values in
Margaret J Holden et al.
Journal of plant physiology, 160(3), 261-269 (2003-05-17)
In Petunia inflata, a species with gametophytic self-incompatibility, pollination triggers two phases of ethylene production by the pistil, the first of which peaks 3 hours after pollination with compatible or incompatible pollen. To investigate the physiological significance of the first
Brenton T Smith et al.
Organic letters, 4(15), 2577-2579 (2002-07-19)
[reaction: see text] The total synthesis of (+)-sparteine was accomplished from 2,5-norbornadione in 15 steps and 15.7% overall yield. The key steps were two ring-expansion reactions, one involving an intramolecular Schmidt reaction and one using a novel variant of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service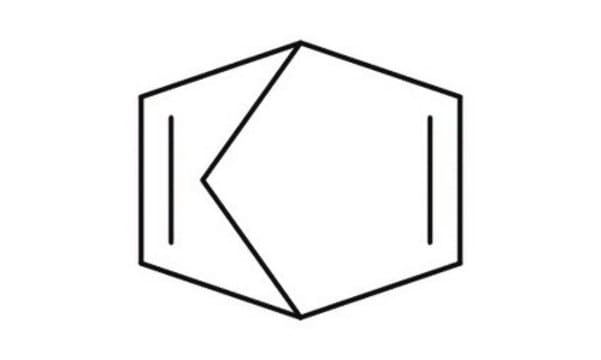
![Bicyclo[2.2.1]hept-2-ene 99%](/deepweb/assets/sigmaaldrich/product/structures/270/492/95fd4909-6108-4858-8c94-609b54387149/640/95fd4909-6108-4858-8c94-609b54387149.png)

![(1S,4S)-2,5-Diphenylbicyclo[2,2,2]octa-2,5-diene 95%](/deepweb/assets/sigmaaldrich/product/structures/313/518/9c1268bf-134a-47cd-81a6-1df7562812d2/640/9c1268bf-134a-47cd-81a6-1df7562812d2.png)