858781
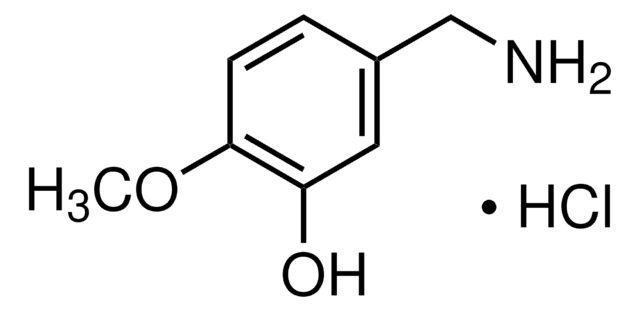
3,4-Dihydroxybenzylamine hydrobromide
98%
Synonym(s):
4-(Aminomethyl)catechol hydrobromide, DHBA hydrobromide
About This Item
Recommended Products
Quality Level
Assay
98%
form
crystals
mp
184-186 °C (lit.)
SMILES string
Br.NCc1ccc(O)c(O)c1
InChI
1S/C7H9NO2.BrH/c8-4-5-1-2-6(9)7(10)3-5;/h1-3,9-10H,4,8H2;1H
InChI key
BVFZTXFCZAXSHN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
<li><strong>Oxidative Polymerization of 3,4-Dihydroxybenzylamine:</strong> 3,4-Dihydroxybenzylamine is used in the synthesis of poly[3,4-dihydroxybenzylamine] (PDHBA) by oxidative polymerization, exploring its application as a lower homolog of dopamine for potential use in synthetic pathways and materials science (Petran et al., 2023).</li>
<li><strong>Detection of Urinary Free Metanephrines:</strong> Utilizing 3,4-Dihydroxybenzylamine hydrobromide as an internal standard, this research enhances the detection accuracy of urinary free metanephrines for diagnosing pheochromocytomas and paragangliomas, showcasing its importance in clinical diagnostic applications (Wang et al., 2020).</li>
<li><strong>Development of HPLC-ECD Method:</strong> A study developed an HPLC-ECD method using 3,4-Dihydroxybenzylamine as an internal standard for the analysis of vitamin C in plasma, demonstrating the chemical’s utility in enhancing analytical methodologies in biochemical research (Clark and Frank, 2016).</li>
<li><strong>Fluorescence Analysis of Catecholamines:</strong> 3,4-Dihydroxybenzylamine is used as an internal standard to determine catecholamines and related compounds in rat brain tissue, underlining its application in neurochemical analysis and research (Fonseca et al., 2017).</li>
</ul>
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service