763705
Graphene oxide dispersion
2 mg/mL,dispersion in H2O, avg. no. of layers, 1
Synonym(s):
graphene oxide aqueous dispersion
About This Item
Recommended Products
product name
Graphene oxide, 2 mg/mL, dispersion in H2O, avg. no. of layers, 1
form
dispersion in H2O
Quality Level
feature
avg. no. of layers 1
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
concentration
2 mg/mL
refractive index
n20/D 1.333
density
0.981 g/mL at 25 °C
greener alternative category
, Enabling
SMILES string
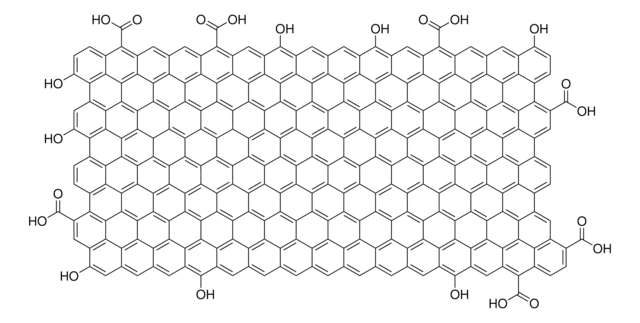
O=C(O)C1C2=C3C4=C5C6=C7C8=C9C%10=C%11C(C%12=C%13C%10=C%14C8=C%15C6=C%16C4=C%17C2=CC(C(O)=O)C%18=C%17C%19=C%16C%20=C%15C%21=C%14C%22=C%13C(C%23=C%24C%22=C%25C%21=C%26C%20=C%27C%19=C%28C%18=CC(C(O)=O)C%29=C%28C%30=C%27C%31=C%26C%32=C%25C%33=C%24C(C%34=C%35C
InChI
1S/C140H42O20/c141-131(142)26-13-23-15-44-62(140(159)160)45-16-24-14-40-31(132(143)144)5-1-29-41-20-48(135(149)150)56-33-7-3-28-27-2-6-32-55-37(133(145)146)11-9-35-60(138(155)156)42-17-25-18-43-61(139(157)158)36-10-12-38(134(147)148)58-46-21-50(137(153)154)59-47-22-49(136(151)152)57-34-8-4-30-39(19-26)51(23)78-72(44)88-75(45)80-52(24)79(54(29)40)95-71(41)83(56)101-93-69(33)64(28)91-90-63(27)68(32)92-86(66(35)55)73(42)81-53(25)82-74(43)87(67(36)58)96-76(46)85(59)103-97-77(47)84(57)102-94-70(34)65(30)89(78)105-104(88)115-98(80)111(95)116(101)126-122-110(93)107(91)120-119-106(90)108(92)99(81)114-100(82)112(96)118(103)128(124(114)119)123-113(97)117(102)127(130(122)129(120)123)121(109(94)105)125(115)126/h2,5,7-10,12-22,26,38,48-50H,1,3-4,6,11H2,(H,141,142)(H,143,144)(H,145,146)(H,147,148)(H,149,150)(H,151,152)(H,153,154)(H,155,156)(H,157,158)(H,159,160)
InChI key
VTWITIAIMADGRM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
- Chloride free (purified by dialysis)
- Monolayer sheet
- sheet diameter <10 micron
Application
It can be used as a support for biocatalysis in organic solvents. For example, carboxyl-functionalized graphene oxide can act as a support to immobilize Yarrowia lipolytica lipase. The immobilized enzyme exhibits a high efficiency for the resolution of the racemic compound in the organic solvent.
It can also be used as a hole transport layer in organic photovoltaic cells.
Features and Benefits
- Good solution processability
- Hydrophilic and easily dispersed in water
- Low production cost
- Presence of rich active oxygen-containing functional groups
- It can be easily functionalized
Preparation Note
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service