All Photos(1)
About This Item
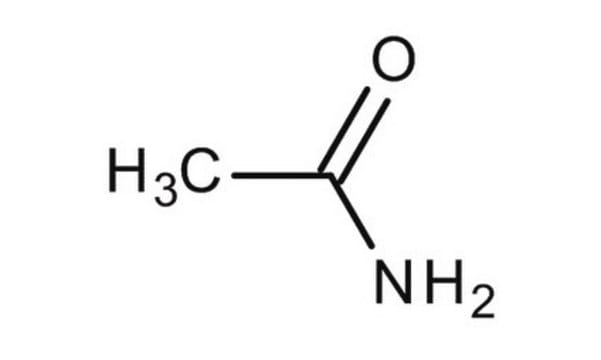
Linear Formula:
CH3CONH2
CAS Number:
Molecular Weight:
59.07
Beilstein:
1071207
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
1 mmHg ( 65 °C)
Assay
99%
form
sublimed
bp
221 °C (lit.)
mp
78-80 °C (lit.)
SMILES string
CC(N)=O
InChI
1S/C2H5NO/c1-2(3)4/h1H3,(H2,3,4)
InChI key
DLFVBJFMPXGRIB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jie Guang et al.
Organic letters, 14(12), 3174-3177 (2012-06-02)
Highly enantioselective aldol reactions of acetylphosphonates and activated carbonyl compounds was realized with cinchona alkaloid derived catalysts, in which the acetylphosphonate was directly used as an enolate precursor for the first time. The aldol product obtained was converted in situ
Lingle Wang et al.
Proceedings of the National Academy of Sciences of the United States of America, 109(6), 1937-1942 (2012-02-07)
We apply a free energy perturbation simulation method, free energy perturbation/replica exchange with solute tempering, to two modifications of protein-ligand complexes that lead to significant conformational changes, the first in the protein and the second in the ligand. The approach
Asal Fallah-Tafti et al.
European journal of medicinal chemistry, 46(10), 4853-4858 (2011-08-20)
KX2-391 (KX-01/Kinex Pharmaceuticals), N-benzyl-2-(5-(4-(2-morpholinoethoxy)phenyl)pyridin-2-yl)acetamide, is a highly selective Src substrate binding site inhibitor. To understand better the role of pyridine ring and N-benzylsubstitution in KX2-391 and establish the structure-activity relationship, a number of N-benzyl substituted (((2-morpholinoethoxy)phenyl)thiazol-4-yl)acetamide derivatives containing thiazole instead
A Subha Mahadevi et al.
Physical chemistry chemical physics : PCCP, 13(33), 15211-15220 (2011-07-16)
Insights into the formation of hydrogen bonded clusters are of outstanding importance and quantum chemical calculations play a pivotal role in achieving this understanding. Structure and energetic comparison of linear, circular and standard forms of (acetamide)(n) clusters (n = 1-15)
E C B Silva et al.
Animal reproduction science, 132(3-4), 155-158 (2012-06-26)
The aim was to assess the in vitro effect of glycerol, ethylene glycol or acetamide on frozen-thawed ram spermatozoa. Aliquots of each sixteen ejaculates from four rams of the Morada Nova breed were diluted in Tris-egg yolk with glycerol (5%)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service